Metastases in the Absence of a Primary Tumor: Advances in the Diagnosis and Treatment of CUP Syndrome
- PMID: 19623297
- PMCID: PMC2696976
- DOI: 10.3238/arztebl.2008.0733
Metastases in the Absence of a Primary Tumor: Advances in the Diagnosis and Treatment of CUP Syndrome
Abstract
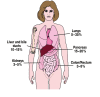
Introduction: The term cancer of unknown primary site (CUP) syndrome is used to describe malignancies in which a complete diagnostic work-up detects metastases in the absence of an identifiable primary tumor.
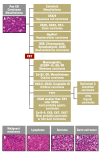
Methods: Based on a selective literature review, national and international guidelines, and the experience of the "Arbeitskreis CUP-Syndrom der Arbeitsgemeinschaft Internistische Onkologie der Deutschen Krebsgesellschaft" (CUP Syndrome Committee of the Medical Oncology Joint Working Group of the German Cancer Society), developments in the diagnosis and treatment of CUP syndrome are reported.
Results: Most patients diagnosed with CUP have an unfavorable prognosis, with a life expectancy of less than 12 months. Nevertheless, it is important to identify subsets of patients in whom specific treatment offers the chance of long-term survival or even full recovery.
Discussion: Only rigorous further development of diagnostic tools and treatment protocols will enable an improvement of the poor prognosis of patients with CUP syndrome. Specific molecular treatment strategies have shown promising results.
Keywords: CUP syndrome; diagnosis; metastasis; molecular medicine; treatment success.
Figures


References
-
- Califano J, Westra WH, Koch W, et al. Unknown primary head and neck squamous cell carcinoma: molecular identification of the site of origin. J Natl Cancer Inst. 1999;91:599–604. - PubMed
-
- Varadhachary GR, Abbruzzese JL, Lenzi R. Diagnostic strategies for unknown primary cancer. Cancer. 2004;100:1776–1785. - PubMed
-
- Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol. 1995;13:2094–2103. - PubMed
-
- Greco FA, Burris HA, Litchy S, et al. Gemcitabine, carboplatin, and paclitaxel for patients with carcinoma of unknown primary site: a Minnie Pearl Cancer Research Network study. J Clin Oncol. 2002;20:1651–1656. - PubMed
-
- Culine S, Lortholary A, Voigt JJ, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study-trial for the French Study Group on Carcinomas of Unknown Primary (GEFCAPI 01) J Clin Oncol. 2003;21:3479–3482. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
