Catalysis of iron core formation in Pyrococcus furiosus ferritin
- PMID: 19623480
- PMCID: PMC2771142
- DOI: 10.1007/s00775-009-0571-z
Catalysis of iron core formation in Pyrococcus furiosus ferritin
Abstract
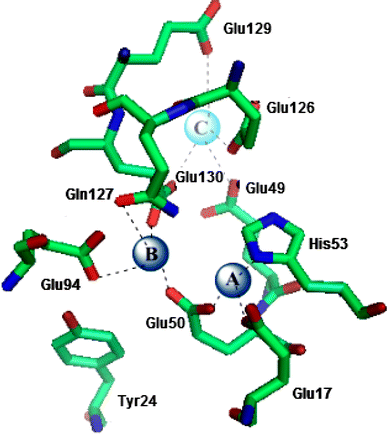
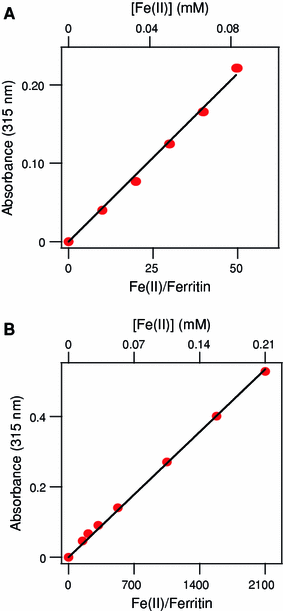
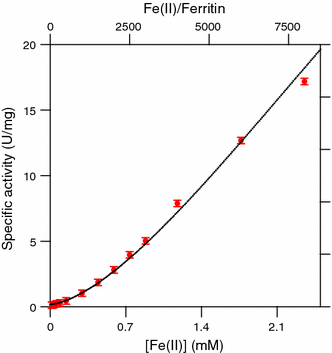
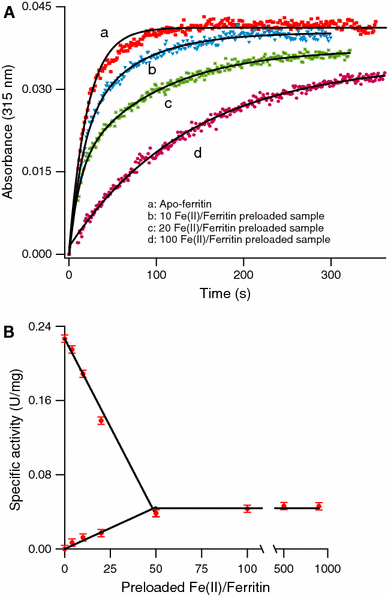
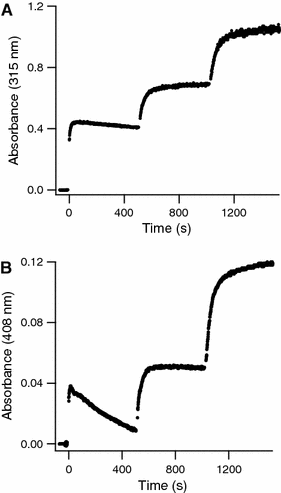
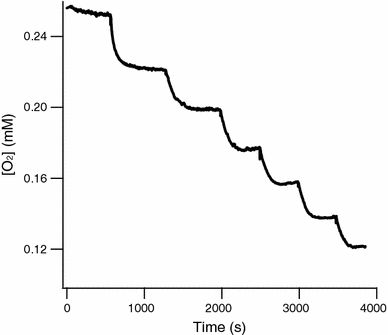
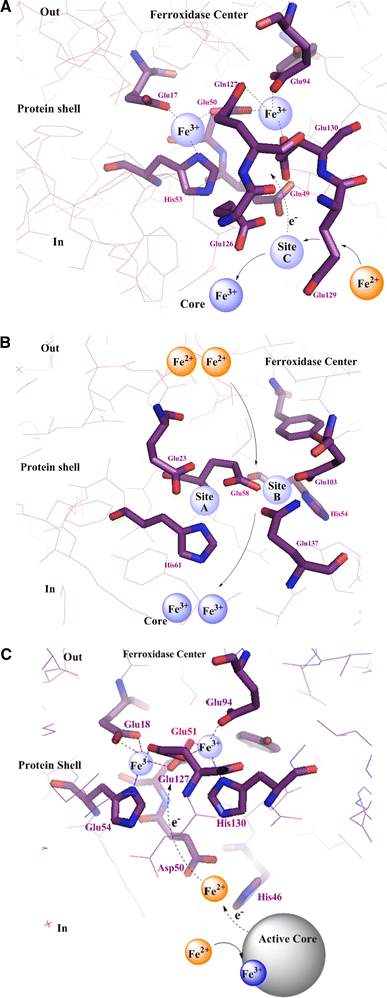
The hollow sphere-shaped 24-meric ferritin can store large amounts of iron as a ferrihydrite-like mineral core. In all subunits of homomeric ferritins and in catalytically active subunits of heteromeric ferritins a diiron binding site is found that is commonly addressed as the ferroxidase center (FC). The FC is involved in the catalytic Fe(II) oxidation by the protein; however, structural differences among different ferritins may be linked to different mechanisms of iron oxidation. Non-heme ferritins are generally believed to operate by the so-called substrate FC model in which the FC cycles by filling with Fe(II), oxidizing the iron, and donating labile Fe(III)-O-Fe(III) units to the cavity. In contrast, the heme-containing bacterial ferritin from Escherichia coli has been proposed to carry a stable FC that indirectly catalyzes Fe(II) oxidation by electron transfer from a core that oxidizes Fe(II). Here, we put forth yet another mechanism for the non-heme archaeal 24-meric ferritin from Pyrococcus furiosus in which a stable iron-containing FC acts as a catalytic center for the oxidation of Fe(II), which is subsequently transferred to a core that is not involved in Fe(II)-oxidation catalysis. The proposal is based on optical spectroscopy and steady-state kinetic measurements of iron oxidation and dioxygen consumption by apoferritin and by ferritin preloaded with different amounts of iron. Oxidation of the first 48 Fe(II) added to apoferritin is spectrally and kinetically different from subsequent iron oxidation and this is interpreted to reflect FC building followed by FC-catalyzed core formation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

