Axin2 regulates chondrocyte maturation and axial skeletal development
- PMID: 19623616
- PMCID: PMC2853598
- DOI: 10.1002/jor.20954
Axin2 regulates chondrocyte maturation and axial skeletal development
Abstract
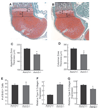
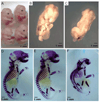
Axis inhibition proteins 1 and 2 (Axin1 and Axin2) are scaffolding proteins that modulate at least two signaling pathways that are crucial in skeletogenesis: the Wnt/beta-catenin and TGF-beta signaling pathways. To determine whether Axin2 is important in skeletogenesis, we examined the skeletal phenotype of Axin2-null mice in a wild-type or Axin1(+/-) background. Animals with disrupted Axin2 expression displayed a runt phenotype when compared to heterozygous littermates. Whole-mount and tissue beta-galactosidase staining of Axin2(LacZ/LacZ) mice revealed that Axin2 is expressed in cartilage tissue, and histological sections from knockout animals showed shorter hypertrophic zones in the growth plate. Primary chondrocytes were isolated from Axin2-null and wild-type mice, cultured, and assayed for type X collagen gene expression. While type II collagen levels were depressed in cells from Axin2-deficient animals, type X collagen gene expression was enhanced. There was no difference in BrdU incorporation between null and heterozygous mice, suggesting that loss of Axin2 does not alter chondrocyte proliferation. Taken together, these findings reveal that disruption of Axin2 expression results in accelerated chondrocyte maturation. In the presence of a heterozygous deficiency of Axin1, Axin2 was also shown to play a critical role in craniofacial and axial skeleton development.
Figures



Similar articles
-
Axin1 and Axin2 are regulated by TGF- and mediate cross-talk between TGF- and Wnt signaling pathways.Ann N Y Acad Sci. 2007 Nov;1116:82-99. doi: 10.1196/annals.1402.082. Ann N Y Acad Sci. 2007. PMID: 18083923 Free PMC article.
-
Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification.J Biol Chem. 2005 May 13;280(19):19185-95. doi: 10.1074/jbc.M414275200. Epub 2005 Mar 10. J Biol Chem. 2005. PMID: 15760903
-
Secreted frizzled related protein 1 regulates Wnt signaling for BMP2 induced chondrocyte differentiation.J Cell Physiol. 2006 Jul;208(1):87-96. doi: 10.1002/jcp.20637. J Cell Physiol. 2006. PMID: 16575902
-
The extended chondrocyte lineage: implications for skeletal homeostasis and disorders.Curr Opin Cell Biol. 2019 Dec;61:132-140. doi: 10.1016/j.ceb.2019.07.011. Epub 2019 Sep 18. Curr Opin Cell Biol. 2019. PMID: 31541943 Review.
-
A fundamental transcription factor for bone and cartilage.Biochem Biophys Res Commun. 2000 Oct 5;276(3):813-6. doi: 10.1006/bbrc.2000.3460. Biochem Biophys Res Commun. 2000. PMID: 11027552 Review.
Cited by
-
Deletion of Axin1 in condylar chondrocytes leads to osteoarthritis-like phenotype in temporomandibular joint via activation of β-catenin and FGF signaling.J Cell Physiol. 2019 Feb;234(2):1720-1729. doi: 10.1002/jcp.27043. Epub 2018 Aug 2. J Cell Physiol. 2019. PMID: 30070692 Free PMC article.
-
Use of integrative epigenetic and mRNA expression analyses to identify significantly changed genes and functional pathways in osteoarthritic cartilage.Bone Joint Res. 2018 Jun 5;7(5):343-350. doi: 10.1302/2046-3758.75.BJR-2017-0284.R1. eCollection 2018 May. Bone Joint Res. 2018. PMID: 29922454 Free PMC article.
-
Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice.Bone. 2014 Sep;66:277-86. doi: 10.1016/j.bone.2014.06.022. Epub 2014 Jun 25. Bone. 2014. PMID: 24973690 Free PMC article.
-
Midkine-deficiency delays chondrogenesis during the early phase of fracture healing in mice.PLoS One. 2014 Dec 31;9(12):e116282. doi: 10.1371/journal.pone.0116282. eCollection 2014. PLoS One. 2014. PMID: 25551381 Free PMC article.
-
Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells.Cells. 2023 Sep 10;12(18):2244. doi: 10.3390/cells12182244. Cells. 2023. PMID: 37759467 Free PMC article.
References
-
- Day TF, Guo X, Garrett-Beal L, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. - PubMed
-
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. - PubMed
-
- Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

