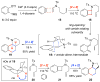
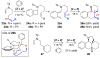
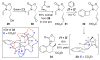
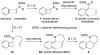Enamide-benzyne-[2 + 2] cycloaddition: stereoselective tandem [2 + 2]-pericyclic ring-opening-intramolecular N-tethered [4 + 2] cycloadditions
- PMID: 19624104
- PMCID: PMC2822464
- DOI: 10.1021/ol901434g
Enamide-benzyne-[2 + 2] cycloaddition: stereoselective tandem [2 + 2]-pericyclic ring-opening-intramolecular N-tethered [4 + 2] cycloadditions
Abstract
Benzyne-[2 + 2] cycloadditions with enamides are described. This effort led to the development of a highly stereoselective tandem [2 + 2] cycloaddition-pericyclic ring-opening-intramolecular-N-tethered-[4 + 2] cycloaddition for rapid assembly of nitrogen heterocycles.
Figures
Similar articles
-
Total syntheses of chelidonine and norchelidonine via an enamide-benzyne-[2 + 2] cycloaddition cascade.Org Lett. 2012 Jun 1;14(11):2742-5. doi: 10.1021/ol300967a. Epub 2012 May 10. Org Lett. 2012. PMID: 22575071 Free PMC article.
-
Thermal intramolecular [4 + 2] cycloadditions of allenamides: a stereoselective tandem propargyl amide isomerization-cycloaddition.Org Lett. 2009 Aug 6;11(15):3430-3. doi: 10.1021/ol901283m. Org Lett. 2009. PMID: 19591454 Free PMC article.
-
Construction of N-Heterocycles Fused with a Highly Substituted Benzene Ring by a Benzyne-Mediated Cyclization/Functionalization Cascade Reaction and Its Application to the Total Synthesis of Marine Natural Products.Chem Pharm Bull (Tokyo). 2021;69(8):707-716. doi: 10.1248/cpb.c21-00389. Chem Pharm Bull (Tokyo). 2021. PMID: 34334514
-
Transition metal-free one-pot synthesis of nitrogen-containing heterocycles.Mol Divers. 2016 Feb;20(1):185-232. doi: 10.1007/s11030-015-9596-0. Epub 2015 Jun 9. Mol Divers. 2016. PMID: 26055184 Review.
-
Cycloaddition chemistry of allenamides.Curr Opin Drug Discov Devel. 2010;13(6):645-57. Curr Opin Drug Discov Devel. 2010. PMID: 21061228 Review.
Cited by
-
Synthesis of dihydrobenzisoxazoles by the [3 + 2] cycloaddition of arynes and oxaziridines.J Org Chem. 2010 Nov 5;75(21):7381-7. doi: 10.1021/jo101656c. J Org Chem. 2010. PMID: 20936802 Free PMC article.
-
Total syntheses of chelidonine and norchelidonine via an enamide-benzyne-[2 + 2] cycloaddition cascade.Org Lett. 2012 Jun 1;14(11):2742-5. doi: 10.1021/ol300967a. Epub 2012 May 10. Org Lett. 2012. PMID: 22575071 Free PMC article.
-
A highly regio- and stereoselective synthesis of α-fluorinated imides via fluorination of chiral enamides.Org Lett. 2015 Feb 6;17(3):572-5. doi: 10.1021/ol503591d. Epub 2015 Jan 12. Org Lett. 2015. PMID: 25582419 Free PMC article.
-
Advances in the synthesis of nitrogen-containing heterocyclic compounds by in situ benzyne cycloaddition.RSC Adv. 2023 Mar 13;13(12):8238-8253. doi: 10.1039/d3ra00400g. eCollection 2023 Mar 8. RSC Adv. 2023. PMID: 36922948 Free PMC article. Review.
-
A Cascade of Strain-Driven Events Converting Benzynes to Alkynylbenzocyclobutenes to 1,3-Dien-5-ynes to Cyclic Allenes to Benzocyclohexadienones.J Am Chem Soc. 2024 Mar 13;146(10):6438-6443. doi: 10.1021/jacs.3c10225. Epub 2024 Mar 4. J Am Chem Soc. 2024. PMID: 38437506 Free PMC article.
References
-
-
For recent reviews on the aryne chemistry, see:
- Peña D, Pérez D, Guitián E. Angew Chem Int Ed. 2006;45:3579. - PubMed
- Pellissier H, Santelli M. Tetrahedron. 2003;59:701.
- Kessar SV. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 4. Pergamon Press; New York: 1991. pp. 483–515.
- Heaney H. Chem Rev. 1962;62:81.
-
-
-
For leading examples of nucleophilic additions, see:
- Liu Z, Larock RC. Org Lett. 2004;6:99. - PubMed
- Jeganmohn M, Chen CH. Org Lett. 2004;6:2821. - PubMed
- Jeganmohan M, Cheng CH. Synthesis. 2005:1693.
- Bhuvaneswari S, Jeganmohan M, Yang MC, Cheng CH. Chem Commun. 2008:2158. - PubMed
- Xie C, Liu L, Zhang Y, Xu P. Org Lett. 2008;10:2393. - PubMed
- Sha F, Huang X. Angew Chem Int Ed. 2009;38:3458. - PubMed
-
-
-
For examples of interesting insertion type processes, see:
- Liu Z, Larock RC. J Am Chem Soc. 2005;127:13112. - PubMed
- Tambar UK, Stoltz BM. J Am Chem Soc. 2005;127:5340. - PubMed
- Tambar UK, Ebner DC, Stoltz BM. J Am Chem Soc. 2006;127:11752. - PMC - PubMed
- Pi SF, Tang BX, Li JH, Liu YL, Liang Y. Org Lett. 2009;11:2309. - PubMed
-
Also see reference 1a
- None
-
-
-
For recent examples on annulations and cycloadditions, see:
- Liu Z, Larock RC. Tetrahedron. 2007;63:347. - PMC - PubMed
- Liu Z, Shi F, Martinez PDG, Raminelli C, Larock RC. J Org Chem. 2008;73:219. - PubMed
- Shi F, Waldo JP, Chen Y, Larock RC. Org Lett. 2008;10:2409. - PMC - PubMed
- Worlikar SA, Larock RC. Org Lett. 2009;11:2413. - PMC - PubMed
-
Also see reference 6
- None
-
-
-
For a mild and general preparation of arynes using ortho-(trimethylsilyl)aryl triflates, see:
- Himeshima Y, Sonoda T, Kobayashi H. Chem Lett. 1983:1211.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources