Prostanoid receptor antagonists: development strategies and therapeutic applications
- PMID: 19624532
- PMCID: PMC2795261
- DOI: 10.1111/j.1476-5381.2009.00317.x
Prostanoid receptor antagonists: development strategies and therapeutic applications
Abstract
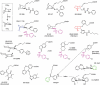
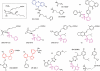
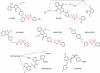
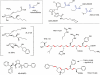
Identification of the primary products of cyclo-oxygenase (COX)/prostaglandin synthase(s), which occurred between 1958 and 1976, was followed by a classification system for prostanoid receptors (DP, EP(1), EP(2) ...) based mainly on the pharmacological actions of natural and synthetic agonists and a few antagonists. The design of potent selective antagonists was rapid for certain prostanoid receptors (EP(1), TP), slow for others (FP, IP) and has yet to be achieved in certain cases (EP(2)). While some antagonists are structurally related to the natural agonist, most recent compounds are 'non-prostanoid' (often acyl-sulphonamides) and have emerged from high-throughput screening of compound libraries, made possible by the development of (functional) assays involving single recombinant prostanoid receptors. Selective antagonists have been crucial to defining the roles of PGD(2) (acting on DP(1) and DP(2) receptors) and PGE(2) (on EP(1) and EP(4) receptors) in various inflammatory conditions; there are clear opportunities for therapeutic intervention. The vast endeavour on TP (thromboxane) antagonists is considered in relation to their limited pharmaceutical success in the cardiovascular area. Correspondingly, the clinical utility of IP (prostacyclin) antagonists is assessed in relation to the cloud hanging over the long-term safety of selective COX-2 inhibitors. Aspirin apart, COX inhibitors broadly suppress all prostanoid pathways, while high selectivity has been a major goal in receptor antagonist development; more targeted therapy may require an intermediate position with defined antagonist selectivity profiles. This review is intended to provide overviews of each antagonist class (including prostamide antagonists), covering major development strategies and current and potential clinical usage.
Figures




Similar articles
-
Characterization of the prostanoid receptor(s) on human blood monocytes at which prostaglandin E2 inhibits lipopolysaccharide-induced tumour necrosis factor-alpha generation.Br J Pharmacol. 1997 Sep;122(1):149-57. doi: 10.1038/sj.bjp.0701360. Br J Pharmacol. 1997. PMID: 9298541 Free PMC article.
-
Prostanoid receptors involved in regulation of the beating rate of neonatal rat cardiomyocytes.PLoS One. 2012;7(9):e45273. doi: 10.1371/journal.pone.0045273. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984630 Free PMC article.
-
In vitro characterization of prostanoid FP-, DP-, IP- and TP-receptors on the non-pregnant human myometrium.Br J Pharmacol. 1992 Sep;107(1):215-21. doi: 10.1111/j.1476-5381.1992.tb14489.x. Br J Pharmacol. 1992. PMID: 1422574 Free PMC article.
-
Prostaglandin FP receptor antagonists: discovery, pharmacological characterization and therapeutic utility.Br J Pharmacol. 2019 Apr;176(8):1059-1078. doi: 10.1111/bph.14335. Epub 2018 Jun 7. Br J Pharmacol. 2019. PMID: 29679483 Free PMC article. Review.
-
International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress.Pharmacol Rev. 2011 Sep;63(3):471-538. doi: 10.1124/pr.110.003517. Epub 2011 Jul 13. Pharmacol Rev. 2011. PMID: 21752876 Review.
Cited by
-
Inverse agonist and pharmacochaperone properties of MK-0524 on the prostanoid DP1 receptor.PLoS One. 2013 Jun 10;8(6):e65767. doi: 10.1371/journal.pone.0065767. Print 2013. PLoS One. 2013. PMID: 23762421 Free PMC article.
-
Interaction of prostanoid EP₃ and TP receptors in guinea-pig isolated aorta: contractile self-synergism of 11-deoxy-16,16-dimethyl PGE₂.Br J Pharmacol. 2011 Jan;162(2):521-31. doi: 10.1111/j.1476-5381.2010.01039.x. Br J Pharmacol. 2011. PMID: 20955363 Free PMC article.
-
Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice.J Cereb Blood Flow Metab. 2017 Jan;37(1):39-51. doi: 10.1177/0271678X15625351. Epub 2016 Jan 8. J Cereb Blood Flow Metab. 2017. PMID: 26746866 Free PMC article.
-
Reciprocal crosstalk between dendritic cells and natural killer cells under the effects of PGE2 in immunity and immunopathology.Cell Mol Immunol. 2013 May;10(3):213-21. doi: 10.1038/cmi.2013.1. Epub 2013 Mar 25. Cell Mol Immunol. 2013. PMID: 23524652 Free PMC article. Review.
-
Molecular biology of histidine decarboxylase and prostaglandin receptors.Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(8):848-66. doi: 10.2183/pjab.86.848. Proc Jpn Acad Ser B Phys Biol Sci. 2010. PMID: 20948178 Free PMC article. Review.
References
-
- Abramovitz M, Boie Y, Nguyen T, Rushmore TH, Bayne MA, Metters KM, et al. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem. 1994;269:2632–2636. - PubMed
-
- Abramovitz M, Adam M, Boie Y, Carrière M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. - PubMed
-
- Ackerley N, Brewster AG, Brown GR, Clarke DS, Foubister AJ, Griffin SJ, et al. A novel approach to dual-acting thromboxane receptor antagonist/synthase inhibitors based on the link of 1,3-dioxane-thromboxane receptor antagonists and thromboxane synthase inhibitors. J Med Chem. 1995;38:1608–1628. - PubMed
-
- Ahn CH, Wallace LJ, Miller DD, Feller DR. Use of [3H]trimetoquinol as a radioligand in human platelets: interaction with putative endoperoxide/thromboxane A2 receptor sites. Thromb Res. 1988;50:387–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

