Compliance of clinical trial registries with the World Health Organization minimum data set: a survey
- PMID: 19624821
- PMCID: PMC2734552
- DOI: 10.1186/1745-6215-10-56
Compliance of clinical trial registries with the World Health Organization minimum data set: a survey
Abstract
Background: Since September 2005 the International Committee of Medical Journal Editors has required that trials be registered in accordance with the World Health Organization (WHO) minimum dataset, in order to be considered for publication. The objective is to evaluate registries' and individual trial records' compliance with the 2006 version of the WHO minimum data set.
Methods: A retrospective evaluation of 21 online clinical trial registries (international, national, specialty, pharmaceutical industry and local) from April 2005 to February 2007 and a cross-sectional evaluation of a stratified random sample of 610 trial records from the 21 registries.
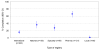
Results: Among 11 registries that provided guidelines for registration, the median compliance with the WHO criteria were 14 out of 20 items (range 6 to 20). In the period April 2005-February 2007, six registries increased their compliance by six data items, on average. None of the local registry websites published guidelines on the trial data items required for registration. Slightly more than half (330/610; 54.1%, 95% CI 50.1% - 58.1%) of trial records completed the contact details criteria while 29.7% (181/610, 95% CI 26.1% - 33.5%) completed the key clinical and methodological data fields.
Conclusion: While the launch of the WHO minimum data set seemed to positively influence registries with better standardisation of approaches, individual registry entries are largely incomplete. Initiatives to ensure quality assurance of registries and trial data should be encouraged. Peer reviewers and editors should scrutinise clinical trial registration records to ensure consistency with WHO's core content requirements when considering trial-related publications.
Figures

References
-
- Levine J, Guy W, Cleary P. Therapeutic trials of psychopharmacologic agents: 1968–1972. Armonk, NY: Futura Publishing Co; 1974.
-
- International collaborative group on clinical trial registries Position paper and consensus recommendations on clinical trial registries. Ad Hoc Working Party of the International Collaborative Group on Clinical Trials Registries. Clin Trials Metaanal. 1993;28:255–266. - PubMed
-
- Deangelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJ, et al. Is this clinical trial fully registered? A statement from the International Committee of Medical Journal Editors. Jama. 2005;293:2927–2929. doi: 10.1001/jama.293.23.jed50037. - DOI - PubMed

