From functional food to medicinal product: systematic approach in analysis of polyphenolics from propolis and wine
- PMID: 19624827
- PMCID: PMC2732638
- DOI: 10.1186/1475-2891-8-33
From functional food to medicinal product: systematic approach in analysis of polyphenolics from propolis and wine
Abstract
In the last decade we have been working on standardization of propolis extract and determination of active constituents of wine those are rich in polyphenolics and have nutritional as well as therapeutic value. Here we are summarizing our results and providing overview on systematic approach how to analyse natural products rich in flavonoids and phenolic acids.Chromatographic methods (thin layer chromatography and high performance liquid chromatography) were used for identification, quantification, and characterization of individual flavonoid or phenolic acid. Total content of active constituents and antioxidant activity were determined by spectrophotometry. Pharmacokinetic parameters were determined by high performance liquid chromatography and using appropriate software. Quantitative structure-activity relationship study of antioxidant activity was conducted, as well as assessment of prolonged propolis supplementation on antioxidative status of organism.Thin layer chromatography-densitometry has been proven as quick and reliable method for standard analysis of propolis and wine; the best mobile phase being chloroform - methanol - formic acid (98-100%) in ratio 44 : 3.5 : 2.5 (v/v). Higher number of polyphenolics was determined by high performance liquid chromatography; 15 compared to 9 by thin layer chromatography. Interactions in situ with acetylsalicylic acid were detected with most of polyphenolics analysed. Plasma protein binding and blood-barrier penetration was greatest for flavone. The interactions with human serum albumin have been grater than 95% for all flavonoids analysed. The prolonged propolis consumption increased superoxide dismutase activity.The necessity of standardization of natural products and their registration as functional nutraceuticals demand easy, quick and inexpensive methods of analysis. In this work we provided overview of analytical part for polyphenolics that could be used as data for possible registration of final products either as functional food or medicinal product.This feature introduces the readers to the authors' research through a concise overview of the selected topic. Reference to important work from others in the field is included.
Figures






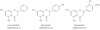
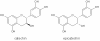

Similar articles
-
Recent advances in the application of high performance liquid chromatography in the analysis of polyphenols in wine and propolis.J AOAC Int. 2011 Jan-Feb;94(1):32-42. J AOAC Int. 2011. PMID: 21391479
-
Advances in the analysis of phenolic compounds in products derived from bees.J Pharm Biomed Anal. 2006 Jun 16;41(4):1220-34. doi: 10.1016/j.jpba.2006.03.002. Epub 2006 Apr 18. J Pharm Biomed Anal. 2006. PMID: 16621403 Review.
-
[Antioxidative activity of propolis from Dalmatia (Croatia)].Acta Med Croatica. 2004;58(5):373-6. Acta Med Croatica. 2004. PMID: 15756802 Croatian.
-
Analytical methodology optimization to estimate the content of non-flavonoid phenolic compounds in Argentine propolis extracts.Pharm Biol. 2014 Jul;52(7):835-40. doi: 10.3109/13880209.2013.871638. Epub 2014 Feb 7. Pharm Biol. 2014. PMID: 24920228
-
The study of phenolic compounds as natural antioxidants in wine.Crit Rev Food Sci Nutr. 2003;43(3):233-44. doi: 10.1080/10408690390826509. Crit Rev Food Sci Nutr. 2003. PMID: 12822671 Review.
Cited by
-
Antiviral effect of methylated flavonol isorhamnetin against influenza.PLoS One. 2015 Mar 25;10(3):e0121610. doi: 10.1371/journal.pone.0121610. eCollection 2015. PLoS One. 2015. PMID: 25806943 Free PMC article.
-
Aloesin as a medical food ingredient for systemic oxidative stress of diabetes.World J Diabetes. 2015 Aug 10;6(9):1097-107. doi: 10.4239/wjd.v6.i9.1097. World J Diabetes. 2015. PMID: 26265996 Free PMC article. Review.
-
Alcohol Consumption Can be a "Double-Edged Sword" for Chronic Kidney Disease Patients.Med Sci Monit. 2019 Sep 20;25:7059-7072. doi: 10.12659/MSM.916121. Med Sci Monit. 2019. PMID: 31538630 Free PMC article. Review.
-
Plasma-initiated graft polymerization of carbon nanoparticles as nano-based drug delivery systems.Biofouling. 2022 Jan;38(1):13-28. doi: 10.1080/08927014.2021.2008376. Epub 2021 Nov 28. Biofouling. 2022. PMID: 34839780 Free PMC article.
-
Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice.J Biol Chem. 2012 Feb 24;287(9):6912-27. doi: 10.1074/jbc.M111.294025. Epub 2012 Jan 4. J Biol Chem. 2012. PMID: 22219198 Free PMC article.
References
-
- Bradamante V, Lacković Z. Oxidative stress and efficiency of antioxidants. Zagreb: Medicinska naklada; 2001.
-
- Smirnoff N. Antioxidants and reactive oxygen species in plants. Oxford: Blackwell Publishing; 2005.
-
- Rastija V, Medić-Šarić M. Polyphenolic composition of Croatian wines with different geographical origins. Food Chem. 2009;115:54–60. doi: 10.1016/j.foodchem.2008.11.071. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

