Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography
- PMID: 19624828
- PMCID: PMC2722582
- DOI: 10.1186/1475-2840-8-39
Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography
Abstract
Background: In vitro data suggest that changes in myocardial substrate metabolism may contribute to impaired myocardial function in diabetic cardiomyopathy (DCM). The purpose of the present study was to study in a rat model of early DCM, in vivo changes in myocardial substrate metabolism and their association with myocardial function.
Methods: Zucker diabetic fatty (ZDF) and Zucker lean (ZL) rats underwent echocardiography followed by [11C]palmitate positron emission tomography (PET) under fasting, and [18F]-2-fluoro-2-deoxy-D-glucose PET under hyperinsulinaemic euglycaemic clamp conditions. Isolated cardiomyocytes were used to determine isometric force development.
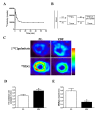
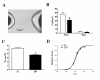
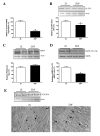
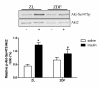
Results: PET data showed a 66% decrease in insulin-mediated myocardial glucose utilisation and a 41% increase in fatty acid (FA) oxidation in ZDF vs. ZL rats (both p < 0.05). Echocardiography showed diastolic and systolic dysfunction in ZDF vs. ZL rats, which was paralleled by a significantly decreased maximal force (68%) and maximal rate of force redevelopment (69%) of single cardiomyocytes. Myocardial functional changes were significantly associated with whole-body insulin sensitivity and decreased myocardial glucose utilisation. ZDF hearts showed a 68% decrease in glucose transporter-4 mRNA expression (p < 0.05), a 22% decrease in glucose transporter-4 protein expression (p = 0.10), unchanged levels of pyruvate dehydrogenase kinase-4 protein expression, a 57% decreased phosphorylation of AMP activated protein kinase alpha1/2 (p < 0.05) and a 2.4-fold increased abundance of the FA transporter CD36 to the sarcolemma (p < 0.01) vs. ZL hearts, which are compatible with changes in substrate metabolism. In ZDF vs. ZL hearts a 2.4-fold reduced insulin-mediated phosphorylation of Akt was found (p < 0.05).
Conclusion: Using PET and echocardiography, we found increases in myocardial FA oxidation with a concomitant decrease of insulin-mediated myocardial glucose utilisation in early DCM. In addition, the latter was associated with impaired myocardial function. These in vivo data expand previous in vitro findings showing that early alterations in myocardial substrate metabolism contribute to myocardial dysfunction.
Figures
Similar articles
-
Diabetic cardiomyopathy in Zucker diabetic fatty rats: the forgotten right ventricle.Cardiovasc Diabetol. 2010 Jun 15;9:25. doi: 10.1186/1475-2840-9-25. Cardiovasc Diabetol. 2010. PMID: 20550678 Free PMC article.
-
Assessment of myocardial metabolism in diabetic rats using small-animal PET: a feasibility study.J Nucl Med. 2006 Apr;47(4):689-97. J Nucl Med. 2006. PMID: 16595504
-
Time course of alterations in myocardial glucose utilization in the Zucker diabetic fatty rat with correlation to gene expression of glucose transporters: a small-animal PET investigation.J Nucl Med. 2008 Aug;49(8):1320-7. doi: 10.2967/jnumed.108.051672. Epub 2008 Jul 16. J Nucl Med. 2008. PMID: 18632819 Free PMC article.
-
Role of changes in cardiac metabolism in development of diabetic cardiomyopathy.Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1489-506. doi: 10.1152/ajpheart.00278.2006. Epub 2006 Jun 2. Am J Physiol Heart Circ Physiol. 2006. PMID: 16751293 Review.
-
A "PET" area of interest: myocardial metabolism in human systolic heart failure.Heart Fail Rev. 2013 Sep;18(5):567-74. doi: 10.1007/s10741-012-9360-9. Heart Fail Rev. 2013. PMID: 23180281 Free PMC article. Review.
Cited by
-
High fat diet-induced glucose intolerance impairs myocardial function, but not myocardial perfusion during hyperaemia: a pilot study.Cardiovasc Diabetol. 2012 Jun 20;11:74. doi: 10.1186/1475-2840-11-74. Cardiovasc Diabetol. 2012. PMID: 22716959 Free PMC article.
-
Prolonged diet induced obesity has minimal effects towards brain pathology in mouse model of cerebral amyloid angiopathy: implications for studying obesity-brain interactions in mice.Biochim Biophys Acta. 2013 Sep;1832(9):1456-62. doi: 10.1016/j.bbadis.2013.01.002. Epub 2013 Jan 9. Biochim Biophys Acta. 2013. PMID: 23313575 Free PMC article.
-
Hyperglycemia-induced stimulation of glucose transport in skeletal muscle measured by PET-[18F]6FDG and [18F]2FDG.Physiol Meas. 2012 Oct;33(10):1661-73. doi: 10.1088/0967-3334/33/10/1661. Epub 2012 Sep 18. Physiol Meas. 2012. PMID: 22986442 Free PMC article.
-
Cardiovascular changes in animal models of metabolic syndrome.J Diabetes Res. 2013;2013:761314. doi: 10.1155/2013/761314. Epub 2013 Mar 14. J Diabetes Res. 2013. PMID: 23691518 Free PMC article.
-
The beneficial effects of exercise in rodents are preserved after detraining: a phenomenon unrelated to GLUT4 expression.Cardiovasc Diabetol. 2010 Oct 28;9:67. doi: 10.1186/1475-2840-9-67. Cardiovasc Diabetol. 2010. PMID: 21029425 Free PMC article.
References
-
- Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta. 2005;1734:112–126. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

