The EP1 subtype of prostaglandin E2 receptor: role in keratinocyte differentiation and expression in non-melanoma skin cancer
- PMID: 19625175
- PMCID: PMC2875085
- DOI: 10.1016/j.plefa.2009.05.025
The EP1 subtype of prostaglandin E2 receptor: role in keratinocyte differentiation and expression in non-melanoma skin cancer
Abstract
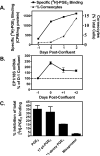
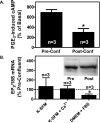
We have previously demonstrated that the EP1 subtype of PGE2 receptor is expressed in the differentiated compartment of normal human epidermis and is coupled to intracellular calcium mobilization. We therefore hypothesized that the EP1 receptor is coupled to keratinocyte differentiation. In in vitro studies, radioligand binding, RT-PCR, immunoblot and receptor agonist-induced second messenger studies demonstrate that the EP1 receptor is up-regulated by high cell density in human keratinocytes and this up-regulation precedes corneocyte formation. Moreover, two different EP1 receptor antagonists, SC51322 and AH6809, both inhibited corneocyte formation. SC51322 also inhibited the induction of differentiation-specific proteins, cytokeratin K10 and epidermal transglutaminase. We next examined the immunolocalization of the EP1 receptor in non-melanoma skin cancer in humans. Well-differentiated SCCs exhibited significantly greater membrane staining, while spindle cell carcinomas and BCCs had significantly decreased membrane staining compared with normal epidermis. This data supports a role for the EP1 receptor in regulating keratinocyte differentiation.
Figures

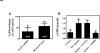
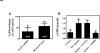
Similar articles
-
The growth of malignant keratinocytes depends on signaling through the PGE(2) receptor EP1.Neoplasia. 2001 Sep-Oct;3(5):402-10. doi: 10.1038/sj.neo.7900182. Neoplasia. 2001. PMID: 11687951 Free PMC article.
-
Human granulosa-lutein cells express functional EP1 and EP2 prostaglandin receptors.Biochem Biophys Res Commun. 2001 Aug 3;285(5):1089-94. doi: 10.1006/bbrc.2001.5301. Biochem Biophys Res Commun. 2001. PMID: 11478765
-
Prostaglandin E receptors in myometrial cells.Acta Physiol Hung. 1997-1998;85(1):39-50. Acta Physiol Hung. 1997. PMID: 9530435
-
Possible cross-regulation of the E prostanoid receptors.Mol Carcinog. 2007 Aug;46(8):711-5. doi: 10.1002/mc.20347. Mol Carcinog. 2007. PMID: 17538953 Review.
-
Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors.Front Biosci. 2004 Sep 1;9:3046-57. doi: 10.2741/1458. Front Biosci. 2004. PMID: 15353336 Review.
Cited by
-
Prostaglandin E receptor EP1 suppresses breast cancer metastasis and is linked to survival differences and cancer disparities.Mol Cancer Res. 2010 Oct;8(10):1310-8. doi: 10.1158/1541-7786.MCR-10-0003. Epub 2010 Sep 21. Mol Cancer Res. 2010. PMID: 20858737 Free PMC article.
-
The EP1 receptor for prostaglandin E2 promotes the development and progression of malignant murine skin tumors.Mol Carcinog. 2012 Jul;51(7):553-64. doi: 10.1002/mc.20820. Epub 2011 Jul 7. Mol Carcinog. 2012. PMID: 21739481 Free PMC article.
-
Metabolic pathways of eicosanoids-derivatives of arachidonic acid and their significance in skin.Cell Mol Biol Lett. 2025 Jan 17;30(1):7. doi: 10.1186/s11658-025-00685-y. Cell Mol Biol Lett. 2025. PMID: 39825220 Free PMC article. Review.
-
Prostaglandin and prostaglandin receptors: present and future promising therapeutic targets for pulmonary arterial hypertension.Respir Res. 2023 Nov 1;24(1):263. doi: 10.1186/s12931-023-02559-3. Respir Res. 2023. PMID: 37915044 Free PMC article. Review.
-
Logical and experimental modeling of cytokine and eicosanoid signaling in psoriatic keratinocytes.iScience. 2021 Nov 15;24(12):103451. doi: 10.1016/j.isci.2021.103451. eCollection 2021 Dec 17. iScience. 2021. PMID: 34877506 Free PMC article.
References
-
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annual Rev Pharmacol Toxicol. 2001;41:661–90. - PubMed
-
- Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin Receptors: Advances in the study of EP3 receptor signaling. J. Biochem. 2002;131:781–784. - PubMed
-
- Abramovitz M, Adam M, Boie Y, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

