Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart
- PMID: 19625257
- PMCID: PMC2785619
- DOI: 10.1074/jbc.M109.028449
Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart
Abstract
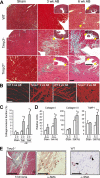
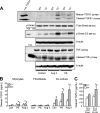
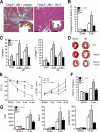
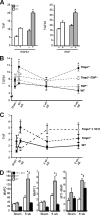
The pleiotropic cytokines, transforming growth factor beta1 (TGFbeta1), and tumor necrosis factor (TNF) play critical roles in tissue homeostasis in response to injury and are implicated in multiple human diseases and cancer. We reported that the loss of Timp3 (tissue inhibitor of metalloproteinase 3) leads to abnormal TNF signaling and cardiovascular function. Here we show that parallel deregulation of TGFbeta1 and TNF signaling in Timp3(-/-) mice amplifies their cross-talk at the onset of cardiac response to mechanical stress (pressure overload), resulting in fibrosis and early heart failure. Microarray analysis showed a distinct gene expression profile in Timp3(-/-) hearts, highlighting activation of TGFbeta1 signaling as a potential mechanism underlying fibrosis. Neonatal cardiomyocyte-cardiofibroblast co-cultures were established to measure fibrogenic response to agonists known to be induced following mechanical stress in vivo. A stronger response occurred in neonatal Timp3(-/-) co-cultures, as determined by increased Smad signaling and collagen expression, due to increased TNF processing and precocious proteolytic maturation of TGFbeta1 to its active form. The relationship between TGFbeta1 and TNF was dissected using genetic and pharmacological manipulations. Timp3(-/-)/Tnf(-/-) mice had lower TGFbeta1 than Timp3(-/-), and anti-TGFbeta1 antibody (1D11) negated the abnormal TNF response, indicating their reciprocal stimulatory effects, with each manipulation abolishing fibrosis and improving heart function. Thus, TIMP3 is a common innate regulator of TGFbeta1 and TNF in tissue response to injury. The matrix-bound TIMP3 balances the anti-inflammatory and proinflammatory processes toward constructive tissue remodeling.
Figures


Similar articles
-
TIMP3/TGF‑β1 axis regulates mechanical loading‑induced chondrocyte degeneration and angiogenesis.Mol Med Rep. 2020 Oct;22(4):2637-2644. doi: 10.3892/mmr.2020.11386. Epub 2020 Jul 29. Mol Med Rep. 2020. PMID: 32945489 Free PMC article.
-
Deficiency of cardiomyocyte-specific microRNA-378 contributes to the development of cardiac fibrosis involving a transforming growth factor β (TGFβ1)-dependent paracrine mechanism.J Biol Chem. 2014 Sep 26;289(39):27199-27215. doi: 10.1074/jbc.M114.580977. Epub 2014 Aug 7. J Biol Chem. 2014. Retraction in: J Biol Chem. 2017 Mar 24;292(12):5124. doi: 10.1074/jbc.A114.580977. PMID: 25104350 Free PMC article. Retracted.
-
Combined effects of interleukin-1α and transforming growth factor-β1 on modulation of human cardiac fibroblast function.Matrix Biol. 2013 Oct-Nov;32(7-8):399-406. doi: 10.1016/j.matbio.2013.03.008. Epub 2013 Apr 12. Matrix Biol. 2013. PMID: 23583823
-
Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities.Mol Aspects Med. 2019 Feb;65:70-99. doi: 10.1016/j.mam.2018.07.001. Epub 2018 Aug 2. Mol Aspects Med. 2019. PMID: 30056242 Review.
-
Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities.J Mol Cell Cardiol. 2016 Jan;90:84-93. doi: 10.1016/j.yjmcc.2015.12.011. Epub 2015 Dec 15. J Mol Cell Cardiol. 2016. PMID: 26705059 Free PMC article. Review.
Cited by
-
Microcurrent stimulation promotes reverse remodelling in cardiomyocytes.ESC Heart Fail. 2016 Jun;3(2):122-130. doi: 10.1002/ehf2.12080. Epub 2016 Jan 6. ESC Heart Fail. 2016. PMID: 27774272 Free PMC article.
-
HNRNPA1-mediated exosomal sorting of miR-483-5p out of renal tubular epithelial cells promotes the progression of diabetic nephropathy-induced renal interstitial fibrosis.Cell Death Dis. 2021 Mar 10;12(3):255. doi: 10.1038/s41419-021-03460-x. Cell Death Dis. 2021. PMID: 33692334 Free PMC article.
-
Multi-label multi-instance transfer learning for simultaneous reconstruction and cross-talk modeling of multiple human signaling pathways.BMC Bioinformatics. 2015 Dec 30;16:417. doi: 10.1186/s12859-015-0841-4. BMC Bioinformatics. 2015. PMID: 26718335 Free PMC article.
-
The Effect of Tissue Inhibitor of Metalloproteinases on Scar Formation after Spinal Cord Injury.Cells. 2024 Sep 14;13(18):1547. doi: 10.3390/cells13181547. Cells. 2024. PMID: 39329731 Free PMC article. Review.
-
Antihypertensive and Antifibrosis Effects of Acupuncture at PC6 Acupoints in Spontaneously Hypertensive Rats and the Underlying Mechanisms.Front Physiol. 2020 Aug 26;11:734. doi: 10.3389/fphys.2020.00734. eCollection 2020. Front Physiol. 2020. PMID: 32982761 Free PMC article.
References
-
- Zile M. R., Baicu C. F., Gaasch W. H. (2004) N. Engl. J. Med. 350, 1953–1959 - PubMed
-
- Katz A. M., Zile M. R. (2006) Circulation 113, 1922–1925 - PubMed
-
- Herpel E., Pritsch M., Koch A., Dengler T. J., Schirmacher P., Schnabel P. A. (2006) Histopathology 48, 736–747 - PubMed
-
- Spinale F. G. (2007) Physiol. Rev. 87, 1285–1342 - PubMed
-
- Stramer B. M., Mori R., Martin P. (2007) J. Invest. Dermatol. 127, 1009–1017 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

