Mutations in the LRRK2 Roc-COR tandem domain link Parkinson's disease to Wnt signalling pathways
- PMID: 19625296
- PMCID: PMC2748899
- DOI: 10.1093/hmg/ddp337
Mutations in the LRRK2 Roc-COR tandem domain link Parkinson's disease to Wnt signalling pathways
Abstract
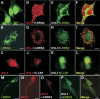
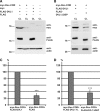
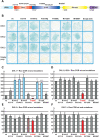
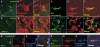
Mutations in PARK8, encoding LRRK2, are the most common known cause of Parkinson's disease. The LRRK2 Roc-COR tandem domain exhibits GTPase activity controlling LRRK2 kinase activity via an intramolecular process. We report the interaction of LRRK2 with the dishevelled family of phosphoproteins (DVL1-3), key regulators of Wnt (Wingless/Int) signalling pathways important for axon guidance, synapse formation and neuronal maintenance. Interestingly, DVLs can interact with and mediate the activation of small GTPases with structural similarity to the LRRK2 Roc domain. The LRRK2 Roc-COR domain and the DVL1 DEP domain were necessary and sufficient for LRRK2-DVL1 interaction. Co-expression of DVL1 increased LRRK2 steady-state protein levels, an effect that was dependent on the DEP domain. Strikingly, LRRK2-DVL1-3 interactions were disrupted by the familial PARK8 mutation Y1699C, whereas pathogenic mutations at residues R1441 and R1728 strengthened LRRK2-DVL1 interactions. Co-expression of DVL1 with LRRK2 in mammalian cells resulted in the redistribution of LRRK2 to typical cytoplasmic DVL1 aggregates in HEK293 and SH-SY5Y cells and co-localization in neurites and growth cones of differentiated dopaminergic SH-SY5Y cells. This is the first report of the modulation of a key LRRK2-accessory protein interaction by PARK8 Roc-COR domain mutations segregating with Parkinson's disease. Since the DVL1 DEP domain is known to be involved in the regulation of small GTPases, we propose that: (i) DVLs may influence LRRK2 GTPase activity, and (ii) Roc-COR domain mutations modulating LRRK2-DVL interactions indirectly influence kinase activity. Our findings also link LRRK2 to Wnt signalling pathways, suggesting novel pathogenic mechanisms and new targets for genetic analysis in Parkinson's disease.
Figures



Similar articles
-
Contribution of GTPase activity to LRRK2-associated Parkinson disease.Small GTPases. 2013 Jul-Sep;4(3):164-70. doi: 10.4161/sgtp.25130. Epub 2013 Jun 10. Small GTPases. 2013. PMID: 24025585 Free PMC article. Review.
-
Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant.J Neurochem. 2011 Jan;116(2):304-15. doi: 10.1111/j.1471-4159.2010.07105.x. J Neurochem. 2011. PMID: 21073465 Free PMC article.
-
Conformational heterogeneity of the Roc domains in C. tepidum Roc-COR and implications for human LRRK2 Parkinson mutations.Biosci Rep. 2015 Aug 26;35(5):e00254. doi: 10.1042/BSR20150128. Biosci Rep. 2015. PMID: 26310572 Free PMC article.
-
Understanding the GTPase Activity of LRRK2: Regulation, Function, and Neurotoxicity.Adv Neurobiol. 2017;14:71-88. doi: 10.1007/978-3-319-49969-7_4. Adv Neurobiol. 2017. PMID: 28353279 Free PMC article. Review.
-
LRRK2 functions as a Wnt signaling scaffold, bridging cytosolic proteins and membrane-localized LRP6.Hum Mol Genet. 2012 Nov 15;21(22):4966-79. doi: 10.1093/hmg/dds342. Epub 2012 Aug 16. Hum Mol Genet. 2012. PMID: 22899650 Free PMC article.
Cited by
-
A proteomic analysis of LRRK2 binding partners reveals interactions with multiple signaling components of the WNT/PCP pathway.Mol Neurodegener. 2017 Jul 11;12(1):54. doi: 10.1186/s13024-017-0193-9. Mol Neurodegener. 2017. PMID: 28697798 Free PMC article.
-
Overview of the Impact of Pathogenic LRRK2 Mutations in Parkinson's Disease.Biomolecules. 2023 May 16;13(5):845. doi: 10.3390/biom13050845. Biomolecules. 2023. PMID: 37238714 Free PMC article. Review.
-
A Wnt1 regulated Frizzled-1/β-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: Therapeutical relevance for neuron survival and neuroprotection.Mol Neurodegener. 2011 Jul 13;6:49. doi: 10.1186/1750-1326-6-49. Mol Neurodegener. 2011. PMID: 21752258 Free PMC article.
-
Neural stem cells in Parkinson's disease: a role for neurogenesis defects in onset and progression.Cell Mol Life Sci. 2015 Feb;72(4):773-97. doi: 10.1007/s00018-014-1774-1. Epub 2014 Nov 18. Cell Mol Life Sci. 2015. PMID: 25403878 Free PMC article. Review.
-
Pathogenic LRRK2 variants are gain-of-function mutations that enhance LRRK2-mediated repression of β-catenin signaling.Mol Neurodegener. 2017 Jan 19;12(1):9. doi: 10.1186/s13024-017-0153-4. Mol Neurodegener. 2017. PMID: 28103901 Free PMC article.
References
-
- Paisán-Ruíz C., Jain S., Evans E.W., Gilks W.P., Simón J., van der Brug M., López de Munain A., Aparicio S., Gil A.M., Khan N., et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. - PubMed
-
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B., et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. - PubMed
-
- Paisán-Ruíz C., Nath P., Washecka N., Gibbs J.R., Singleton A.B. Comprehensive analysis of LRRK2 in publicly available Parkinson's disease cases and neurologically normal controls. Hum. Mutat. 2008;29:485–490. - PubMed
-
- Khan N.L., Jain S., Lynch J.M., Pavese N., Abou-Sleiman P., Holton J.L., Healy D.G., Gilks W.P., Sweeney M.G., Ganguly M., et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

