Algorithm combining toxin immunoassay and stool culture for diagnosis of Clostridium difficile infection
- PMID: 19625481
- PMCID: PMC2738110
- DOI: 10.1128/JCM.00609-09
Algorithm combining toxin immunoassay and stool culture for diagnosis of Clostridium difficile infection
Abstract
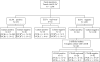
Enzyme immunoassays (EIA) to detect glutamate dehydrogenase or toxins A (TcdA) and B (TcdB), a cytotoxicity assay, and bacteriologic culture have disadvantages when applied individually to diagnosis of Clostridium difficile infections. Stool specimens (n = 1,596) were subjected to toxin detection via an enzyme-linked fluorescent immunoassay (ELFA; Vidas CDAB assay) and bacteriologic culture for toxigenic C. difficile in a three-step algorithm with additional toxigenic culture. Isolates (n = 163) from ELFA-negative stool specimens were examined via ELFA for toxin production. We amplified tcdA and tcdB from C. difficile isolates and tcdB from stool specimens that were ELFA positive or equivocal and culture negative, and we compared the results to those obtained with the three-step algorithm. More than 26% of stool specimens (419/1,596) were culture positive, yielding 248 isolates (59.2%) with both toxin genes (tcdA- and tcdB-positive isolates), 88 isolates (21.0%) with either tcdA or tcdB, and 83 (19.8%) that had no toxin genes (tcdA- and tcdB-negative isolates). Among 49 (culture-negative/ELFA-positive or -equivocal) stool specimens, 53.1% (26/49) represented tcdB-positive isolates. Therefore, the total number of PCR-positive cases was 362, and 27.1% (98/362) of these were detected through toxigenic culture. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 63.3%, 96.7%, 90.5%, and 92.4% (ELFA alone); 92.8%, 93.3%, 80.2%, and 97.8% (culture); and 70.7%, 91.4%, 95.5%, and 100% (three-step algorithm ELFA and bacterial culture with toxigenic culture), respectively, with culture and PCR for tcdA and tcdB as the standards. Thus, sensitivity and specificity were highest using culture and ELFA, respectively, but we recommend the three-step algorithm comprising EIA to detect both toxins and toxigenic culture for C. difficile as a practical method for achieving better PPV and NPV.
Figures
References
-
- al-Barrak, A., J. M. Embil, B. Dyck, K. Olekson, D. Nicoll, M. Alfa, and A. Kabani. 1999. An outbreak of toxin A negative, toxin B positive Clostridium difficile-associated diarrhea in a Canadian tertiary-care hospital. Can. Commun. Dis. Rep. 2565-69. - PubMed
-
- Aldeen, W. E., M. Bingham, A. Aiderzada, J. Kucera, S. Jense, and K. C. Carroll. 2000. Comparison of the Tox A/B test to a cell culture cytotoxicity assay for the detection of Clostridium difficile in stools. Diagn. Microbiol. Infect. Dis. 36211-213. - PubMed
-
- Alfa, M. J., A. Kabani, D. Lyerly, S. Moncrief, L. M. Neville, A. al-Barrack, G. K. H. Harding, B. Dyck, K. Olekson, and J. M. Embil. 2000. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. J. Clin. Microbiol. 382706-2714. - PMC - PubMed
-
- Anderson, M. A., and J. G. Songer. 2008. Evaluation of two enzyme immunoassay for detection of Clostridium difficile toxin A and B in swine. Vet. Microbiol. 128204-206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical