Inhibition of ATR protein kinase activity by schisandrin B in DNA damage response
- PMID: 19625493
- PMCID: PMC2761266
- DOI: 10.1093/nar/gkp593
Inhibition of ATR protein kinase activity by schisandrin B in DNA damage response
Abstract
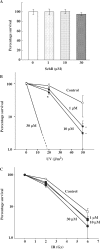
ATM and ATR protein kinases play a crucial role in cellular DNA damage responses. The inhibition of ATM and ATR can lead to the abolition of the function of cell cycle checkpoints. In this regard, it is expected that checkpoint inhibitors can serve as sensitizing agents for anti-cancer chemo/radiotherapy. Although several ATM inhibitors have been reported, there are no ATR-specific inhibitors currently available. Here, we report the inhibitory effect of schisandrin B (SchB), an active ingredient of Fructus schisandrae, on ATR activity in DNA damage response. SchB treatment significantly decreased the viability of A549 adenocarcinoma cells after UV exposure. Importantly, SchB treatment inhibited both the phosphorylation levels of ATM and ATR substrates, as well as the activity of the G2/M checkpoint in UV-exposed cells. The protein kinase activity of immunoaffinity-purified ATR was dose-dependently decreased by SchB in vitro (IC(50): 7.25 muM), but the inhibitory effect was not observed in ATM, Chk1, PI3K, DNA-PK, and mTOR. The extent of UV-induced phosphorylation of p53 and Chk1 was markedly reduced by SchB in ATM-deficient but not siATR-treated cells. Taken together, our demonstration of the ability of SchB to inhibit ATR protein kinase activity following DNA damage in cells has clinical implications in anti-cancer therapy.
Figures






References
-
- Shiloh Y. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 2001;11:71–77. - PubMed
-
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. - PubMed
-
- Collis SJ, Swartz MJ, Nelson WG, DeWeese TL. Enhanced radiation and chemotherapy-mediated cell killing of human cancer cells by small inhibitory RNA silencing of DNA repair factors. Cancer Res. 2003;63:1550–1554. - PubMed
-
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

