Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas
- PMID: 19625505
- PMCID: PMC6665563
- DOI: 10.1523/JNEUROSCI.5478-08.2009
Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas
Abstract
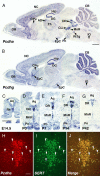
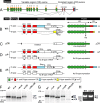
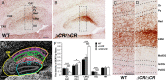
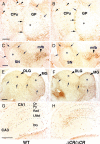
Serotonergic axons from the raphe nuclei in the brainstem project to every region of the brain, where they make connections through their extensive terminal arborizations. This serotonergic innervation contributes to various normal behaviors and psychiatric disorders. The protocadherin-alpha (Pcdha) family of clustered protocadherins consists of 14 cadherin-related molecules generated from a single gene cluster. We found that the Pcdhas were strongly expressed in the serotonergic neurons. To elucidate their roles, we examined serotonergic fibers in a mouse mutant (Pcdha(Delta CR/Delta CR)) lacking the Pcdha cytoplasmic region-encoding exons, which are common to the gene cluster. In the first week after birth, the distribution pattern of serotonergic fibers in Pcdha(Delta CR/Delta CR) mice was similar to wild-type, but by 3 weeks of age, when the serotonergic axonal termini complete their arborizations, the distribution of the projections was abnormal. In some target regions, notably the globus pallidus and substantia nigra, the normally even distribution of serotonin axonal terminals was, in the mutants, dense at the periphery of each region, but sparse in the center. In the stratum lacunosum-molecular of the hippocampus, the mutants showed denser serotonergic innervation than in wild-type, and in the dentate gyrus of the hippocampus and the caudate-putamen, the innervation was sparser. Together, the abnormalities suggested that Pcdha proteins are important in the late-stage maturation of serotonergic projections. Further examination of alternatively spliced exons encoding the cytoplasmic tail showed that the A-type (but not the B-type) cytoplasmic tail was essential for the normal development of serotonergic projections.
Figures




References
-
- Agarwala KL, Nakamura S, Tsutsumi Y, Yamakawa K. Down syndrome cell adhesion molecule DSCAM mediates homophilic intercellular adhesion. Brain Res Mol Brain Res. 2000;79:118–126. - PubMed
-
- Azmitia EC, Dolan K, Whitaker-Azmitia PM. S-100B but not NGF, EGF, insulin or calmodulin is a CNS serotonergic growth factor. Brain Res. 1990;516:354–356. - PubMed
-
- Baumgarten HG, Grozdanovic Z. Psychopharmacology of central serotonergic systems. Pharmacopsychiatry. 1995;28(Suppl 2):73–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
