Reduced amyloid deposition in mice overexpressing RTN3 is adversely affected by preformed dystrophic neurites
- PMID: 19625507
- PMCID: PMC2743151
- DOI: 10.1523/JNEUROSCI.5741-08.2009
Reduced amyloid deposition in mice overexpressing RTN3 is adversely affected by preformed dystrophic neurites
Abstract
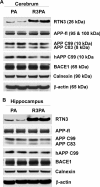
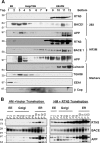
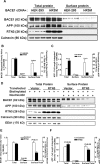
Reticulon 3 (RTN3) was initially identified as a negative modulator of BACE1, an enzyme that cleaves amyloid precursor protein (APP) to release beta-amyloid peptide. Interestingly, RTN3 can also form aggregates after accumulation, and increased RTN3 aggregation correlates with the formation of RTN3 immunoreactive dystrophic neurites (RIDNs) in brains of Alzheimer's cases. Transgenic mice expressing RTN3 alone develop RIDNs in their hippocampus but not in their cortex. To determine the in vivo effects of RTN3 and preformed RIDNs on amyloid deposition, we crossed bitransgenic mice expressing APP and presenilin 1 (PS1) mutations with mice overexpressing RTN3. We found that amyloid deposition in cortex, the hippocampal CA3 region, and dentate gyrus was significantly reduced in triple transgenic mice compared with bitransgenic controls. However, reduction of amyloid deposition in the hippocampal CA1 region, where RIDNs predominantly formed before amyloid deposition, was less significant. Hence, preformed RTN3 aggregates in RIDNs clearly offset the negative modulation of BACE1 activity by RTN3. Furthermore, our study indicates that the increased expression of RTN3 could result in an alteration of BACE1 intracellular trafficking by retaining more BACE1 in the endoplasmic reticulum compartment where cleavage of APP by BACE1 is less favored. Our results suggest that inhibition of RTN3 aggregation is likely to be beneficial by reducing both amyloid deposition and the formation RIDNs.
Figures




Similar articles
-
Reduction of β-amyloid accumulation by reticulon 3 in transgenic mice.Curr Alzheimer Res. 2013 Feb;10(2):135-42. doi: 10.2174/1567205011310020003. Curr Alzheimer Res. 2013. PMID: 22742855
-
Preventing formation of reticulon 3 immunoreactive dystrophic neurites improves cognitive function in mice.J Neurosci. 2013 Feb 13;33(7):3059-66. doi: 10.1523/JNEUROSCI.2445-12.2013. J Neurosci. 2013. PMID: 23407961 Free PMC article.
-
Increased expression of reticulon 3 in neurons leads to reduced axonal transport of β site amyloid precursor protein-cleaving enzyme 1.J Biol Chem. 2013 Oct 18;288(42):30236-30245. doi: 10.1074/jbc.M113.480079. Epub 2013 Sep 4. J Biol Chem. 2013. PMID: 24005676 Free PMC article.
-
Effects of altered RTN3 expression on BACE1 activity and Alzheimer's neuritic plaques.Rev Neurosci. 2017 Feb 1;28(2):145-154. doi: 10.1515/revneuro-2016-0054. Rev Neurosci. 2017. PMID: 27883331 Review.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
-
Diabetes-induced central neuritic dystrophy and cognitive deficits are associated with the formation of oligomeric reticulon-3 via oxidative stress.J Biol Chem. 2013 May 31;288(22):15590-9. doi: 10.1074/jbc.M112.440784. Epub 2013 Apr 16. J Biol Chem. 2013. PMID: 23592790 Free PMC article.
-
ER Dynamics and Derangement in Neurological Diseases.Front Neurosci. 2018 Feb 20;12:91. doi: 10.3389/fnins.2018.00091. eCollection 2018. Front Neurosci. 2018. PMID: 29515361 Free PMC article. Review.
-
FGF21 modulates hippocampal cold-shock proteins and CA2-subregion proteins in neonatal mice with hypoxia-ischemia.Pediatr Res. 2023 Oct;94(4):1355-1364. doi: 10.1038/s41390-023-02652-9. Epub 2023 May 16. Pediatr Res. 2023. PMID: 37193753 Free PMC article.
-
The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential.J Neurosci. 2009 Oct 14;29(41):12787-94. doi: 10.1523/JNEUROSCI.3657-09.2009. J Neurosci. 2009. PMID: 19828790 Free PMC article. Review.
-
Mechanistic Insights into Selective Autophagy Subtypes in Alzheimer's Disease.Int J Mol Sci. 2022 Mar 25;23(7):3609. doi: 10.3390/ijms23073609. Int J Mol Sci. 2022. PMID: 35408965 Free PMC article. Review.
References
-
- Bandtlow CE, Dlaska M, Pirker S, Czech T, Baumgartner C, Sperk G. Increased expression of Nogo-A in hippocampal neurons of patients with temporal lobe epilepsy. Eur J Neurosci. 2004;20:195–206. - PubMed
-
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
-
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. - PubMed
-
- Creemers JW, Ines Dominguez D, Plets E, Serneels L, Taylor NA, Multhaup G, Craessaerts K, Annaert W, De Strooper B. Processing of beta-secretase by furin and other members of the proprotein convertase family. J Biol Chem. 2001;276:4211–4217. - PubMed
-
- Dickson TC, King CE, McCormack GH, Vickers JC. Neurochemical diversity of dystrophic neurites in the early and late stages of Alzheimer's disease. Exp Neurol. 1999;156:100–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
