Slowly adapting mechanoreceptors in the borders of the human fingernail encode fingertip forces
- PMID: 19625527
- PMCID: PMC6665555
- DOI: 10.1523/JNEUROSCI.0143-09.2009
Slowly adapting mechanoreceptors in the borders of the human fingernail encode fingertip forces
Abstract
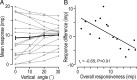
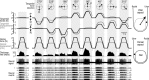
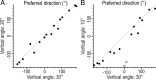
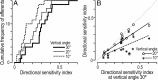
There are clusters of slowly adapting (SA) mechanoreceptors in the skin folds bordering the nail. These "SA-IInail" afferents, which constitute nearly one fifth of the tactile afferents innervating the fingertip, possess the general discharge characteristics of slowly adapting type II (SA-II) tactile afferents located elsewhere in the glabrous skin of the human hand. Little is known about the signals in the SA-IInail afferents when the fingertips interact with objects. Here we show that SA-IInail afferents reliably respond to fingertip forces comparable to those arising in everyday manipulations. Using a flat stimulus surface, we applied forces to the finger pad while recording impulse activity in 17 SA-IInail afferents. Ramp-and-hold forces (amplitude 4 N, rate 10 N/s) were applied normal to the skin, and at 10, 20, or 30 degrees from the normal in eight radial directions with reference to the primary site of contact (25 force directions in total). All afferents responded to the force stimuli, and the responsiveness of all but one afferents was broadly tuned to a preferred direction of force. The preferred directions among afferents were distributed all around the angular space, suggesting that the population of SA-IInail afferents could encode force direction. We conclude that signals in the population of SA-IInail afferents terminating in the nail walls contain vectorial information about fingertip forces. The particular tactile features of contacted surfaces would less influence force-related signals in SA-IInail afferents than force-related signals present in afferents terminating in the volar skin areas that directly contact objects.
Figures






References
-
- Ashbell TS, Kleinert HE, Putcha SM, Kutz JE. The deformed finger nail, a frequent result of failure to repair nail bed injuries. J Trauma. 1967;7:177–190. - PubMed
-
- Bisley JW, Goodwin AW, Wheat HE. Slowly adapting type I afferents from the sides and end of the finger respond to stimuli on the centre of the fingerpad. J Neurophysiol. 2000;84:57–64. - PubMed
-
- Burgess PR, Perl ER. Cutaneous mechanoreceptors and nociceptors. In: Iggo A, editor. Handbook of sensory physiology. Berlin: Springer; 1973. pp. 30–78.
-
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
