Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus
- PMID: 19625534
- PMCID: PMC2746788
- DOI: 10.1152/jn.90695.2008
Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus
Abstract
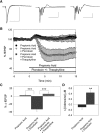
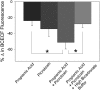
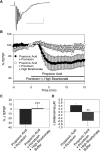
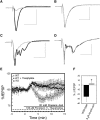
Decreased pH increases extracellular adenosine in CNS regions as diverse as hippocampus and ventral medulla. However, thus far there is no clear consensus whether the critical pH change is a decrease in intracellular and/or extracellular pH. Previously we showed that a decrease in extracellular pH is necessary and a decrease in intracellular pH alone is not sufficient, to increase extracellular adenosine in an acute hippocampal slice preparation. Here we explored further the role of intracellular pH under different synaptic conditions in the hippocampal slice. When synaptic excitability was increased, either during gamma-aminobutyric acid type A receptor blockade in CA1 or after the induction of persistent bursting in CA3, a decrease in intracellular pH alone was now sufficient to: 1) elevate extracellular adenosine concentration, 2) activate adenosine A1 receptors, 3) decrease excitatory synaptic transmission (CA1), and 4) attenuate burst frequency in an in vitro seizure model (CA3). Hippocampal slices obtained from adenosine A1 receptor knockout mice did not exhibit these pH-mediated effects on synaptic transmission, further confirming the role of adenosine acting at the adenosine A1 receptor. Taken together, these data strengthen and add significantly to the evidence outlining a change in pH as an important stimulus influencing extracellular adenosine. In addition, we identify conditions under which intracellular pH plays a dominant role in regulating extracellular adenosine concentrations.
Figures
Similar articles
-
C-Jun N-terminal kinase regulates adenosine A1 receptor-mediated synaptic depression in the rat hippocampus.Neuropharmacology. 2007 Dec;53(8):906-17. doi: 10.1016/j.neuropharm.2007.09.001. Epub 2007 Sep 20. Neuropharmacology. 2007. PMID: 17967469
-
The role of extracellular adenosine in regulating mossy fiber synaptic plasticity.J Neurosci. 2005 Mar 16;25(11):2832-7. doi: 10.1523/JNEUROSCI.4260-04.2005. J Neurosci. 2005. PMID: 15772343 Free PMC article.
-
Frequency facilitation at mossy fiber-CA3 synapses of freely behaving rats is regulated by adenosine A1 receptors.J Neurosci. 2008 Apr 30;28(18):4836-40. doi: 10.1523/JNEUROSCI.3729-07.2008. J Neurosci. 2008. PMID: 18448660 Free PMC article.
-
Regulation of muscarinic acetylcholine receptor-mediated synaptic responses by adenosine receptors in the rat hippocampus.J Physiol. 1997 Jul 1;502 ( Pt 1)(Pt 1):75-90. doi: 10.1111/j.1469-7793.1997.075bl.x. J Physiol. 1997. PMID: 9234198 Free PMC article.
-
Adenosine receptor antagonists induce persistent bursting in the rat hippocampal CA3 region via an NMDA receptor-dependent mechanism.J Neurophysiol. 2000 Apr;83(4):1787-95. doi: 10.1152/jn.2000.83.4.1787. J Neurophysiol. 2000. PMID: 10758091
Cited by
-
Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors.J Neurosci. 2010 Jan 13;30(2):545-55. doi: 10.1523/JNEUROSCI.4920-09.2010. J Neurosci. 2010. PMID: 20071517 Free PMC article.
-
Depolarizing actions of GABA in immature neurons depend neither on ketone bodies nor on pyruvate.J Neurosci. 2011 Jan 5;31(1):34-45. doi: 10.1523/JNEUROSCI.3314-10.2011. J Neurosci. 2011. PMID: 21209187 Free PMC article.
-
Emerging roles of Na⁺/H⁺ exchangers in epilepsy and developmental brain disorders.Prog Neurobiol. 2016 Mar-May;138-140:19-35. doi: 10.1016/j.pneurobio.2016.02.002. Epub 2016 Mar 8. Prog Neurobiol. 2016. PMID: 26965387 Free PMC article. Review.
-
Purines and the Anti-Epileptic Actions of Ketogenic Diets.Open Neurosci J. 2010 Jan 1;4(1):58-63. doi: 10.2174/1874082001004010058. Open Neurosci J. 2010. PMID: 22064941 Free PMC article.
-
Diminishing neuronal acidification by channelrhodopsins with low proton conduction.bioRxiv [Preprint]. 2023 Sep 14:2023.02.07.527404. doi: 10.1101/2023.02.07.527404. bioRxiv. 2023. Update in: Elife. 2023 Oct 06;12:RP86833. doi: 10.7554/eLife.86833. PMID: 36798192 Free PMC article. Updated. Preprint.
References
-
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci 2: 720–726, 1999. - PubMed
-
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci 8: 1560–1567, 2005. - PubMed
-
- Boison D. Adenosine as a modulator of brain activity. Drug News Perspect 20: 607–611, 2007. - PubMed
-
- Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem 67: 2282–2291, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

