Stable encoding of task structure coexists with flexible coding of task events in sensorimotor striatum
- PMID: 19625536
- PMCID: PMC2775375
- DOI: 10.1152/jn.00522.2009
Stable encoding of task structure coexists with flexible coding of task events in sensorimotor striatum
Abstract
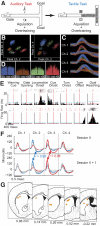
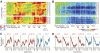
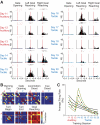
The sensorimotor striatum, as part of the brain's habit circuitry, has been suggested to store fixed action values as a result of stimulus-response learning and has been contrasted with a more flexible system that conditionally assigns values to behaviors. The stability of neural activity in the sensorimotor striatum is thought to underlie not only normal habits but also addiction and clinical syndromes characterized by behavioral fixity. By recording in the sensorimotor striatum of mice, we asked whether neuronal activity acquired during procedural learning would be stable even if the sensory stimuli triggering the habitual behavior were altered. Contrary to expectation, both fixed and flexible activity patterns appeared. One, representing the global structure of the acquired behavior, was stable across changes in task cuing. The second, a fine-grain representation of task events, adjusted rapidly. Such dual forms of representation may be critical to allow motor and cognitive flexibility despite habitual performance.
Figures









Similar articles
-
Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories.Nature. 2005 Oct 20;437(7062):1158-61. doi: 10.1038/nature04053. Nature. 2005. PMID: 16237445
-
Building neural representations of habits.Science. 1999 Nov 26;286(5445):1745-9. doi: 10.1126/science.286.5445.1745. Science. 1999. PMID: 10576743
-
Differential corticostriatal plasticity during fast and slow motor skill learning in mice.Curr Biol. 2004 Jul 13;14(13):1124-34. doi: 10.1016/j.cub.2004.06.053. Curr Biol. 2004. PMID: 15242609
-
Auditory perceptual learning and changes in the conceptualization of auditory cortex.Hear Res. 2018 Sep;366:3-16. doi: 10.1016/j.heares.2018.03.011. Epub 2018 Mar 12. Hear Res. 2018. PMID: 29551308 Review.
-
Habit learning and memory in mammals: behavioral and neural characteristics.Neurobiol Learn Mem. 2014 Oct;114:198-208. doi: 10.1016/j.nlm.2014.06.010. Epub 2014 Jun 26. Neurobiol Learn Mem. 2014. PMID: 24981854 Review.
Cited by
-
Using optogenetics to study habits.Brain Res. 2013 May 20;1511:102-14. doi: 10.1016/j.brainres.2013.01.008. Epub 2013 Jan 10. Brain Res. 2013. PMID: 23313580 Free PMC article. Review.
-
Spatially restricted inhibition of cholinergic interneurons in the dorsolateral striatum encourages behavioral exploration.Eur J Neurosci. 2021 Apr;53(8):2567-2579. doi: 10.1111/ejn.15117. Epub 2021 Feb 14. Eur J Neurosci. 2021. PMID: 33462844 Free PMC article.
-
The striatum: where skills and habits meet.Cold Spring Harb Perspect Biol. 2015 Aug 3;7(8):a021691. doi: 10.1101/cshperspect.a021691. Cold Spring Harb Perspect Biol. 2015. PMID: 26238359 Free PMC article. Review.
-
Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value.Nat Neurosci. 2012 Sep;15(9):1281-9. doi: 10.1038/nn.3188. Epub 2012 Aug 19. Nat Neurosci. 2012. PMID: 22902719 Free PMC article.
-
Many hats: intratrial and reward level-dependent BOLD activity in the striatum and premotor cortex.J Neurophysiol. 2013 Oct;110(7):1689-702. doi: 10.1152/jn.00164.2012. Epub 2013 Jun 5. J Neurophysiol. 2013. PMID: 23741040 Free PMC article.
References
-
- Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can J Physiol Pharmacol 82: 732–739, 2004 - PubMed
-
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol 499: 33–48, 2006 - PubMed
-
- Atallah HE, Lopez-Paniagua D, Rudy JW, O'Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci 10: 126–131, 2007 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

