Encoding and decoding of learned smooth-pursuit eye movements in the floccular complex of the monkey cerebellum
- PMID: 19625543
- PMCID: PMC2775373
- DOI: 10.1152/jn.00075.2009
Encoding and decoding of learned smooth-pursuit eye movements in the floccular complex of the monkey cerebellum
Abstract
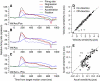
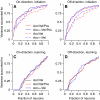
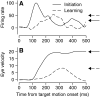
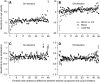
We recorded the simple-spike (SS) firing of Purkinje cells (PCs) in the floccular complex both during normal pursuit caused by step-ramp target motions and after learning induced by a consistently timed change in the direction of target motion. The encoding of eye movement by the SS firing rate of individual PCs was described by a linear regression model, in which the firing rate is a sum of weighted components related to eye acceleration, velocity, and position. Although the model fit the data well for individual conditions, the regression coefficients for the learned component of firing often differed substantially from those for normal pursuit of step-ramp target motion. We suggest that the different encoding of learned versus normal pursuit responses in individual PCs reflects different amounts of learning in their inputs. The decoded output from the floccular complex, estimated by averaging responses across the population of PCs, also was fitted by the regression model. Regression coefficients were equal for the two conditions for on-direction pursuit, but differed for off-direction target motion. We conclude that the average output from the population of floccular PCs provides some, but not all, of the neural signals that drive the learned component of pursuit and that plasticity outside of the flocculus makes an important contribution.
Figures









Similar articles
-
Changes in the responses of Purkinje cells in the floccular complex of monkeys after motor learning in smooth pursuit eye movements.J Neurophysiol. 2000 Dec;84(6):2945-60. doi: 10.1152/jn.2000.84.6.2945. J Neurophysiol. 2000. PMID: 11110823 Free PMC article.
-
Responses of Purkinje cells in the oculomotor vermis of monkeys during smooth pursuit eye movements and saccades: comparison with floccular complex.J Neurophysiol. 2017 Aug 1;118(2):986-1001. doi: 10.1152/jn.00209.2017. Epub 2017 May 17. J Neurophysiol. 2017. PMID: 28515286 Free PMC article.
-
Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes.J Neurophysiol. 1990 May;63(5):1241-61. doi: 10.1152/jn.1990.63.5.1241. J Neurophysiol. 1990. PMID: 2358872
-
Complex predictive eye pursuit in monkey: a model system for cerebellar studies of skilled movement.Arch Ital Biol. 2002 Oct;140(4):331-40. Arch Ital Biol. 2002. PMID: 12228986 Review.
-
Internal models of eye movement in the floccular complex of the monkey cerebellum.Neuroscience. 2009 Sep 1;162(3):763-76. doi: 10.1016/j.neuroscience.2009.03.059. Epub 2009 Mar 29. Neuroscience. 2009. PMID: 19336251 Free PMC article. Review.
Cited by
-
A cerebellar adaptation to uncertain inputs.Sci Adv. 2018 May 30;4(5):eaap9660. doi: 10.1126/sciadv.aap9660. eCollection 2018 May. Sci Adv. 2018. PMID: 29854943 Free PMC article.
-
Computational Principles of Supervised Learning in the Cerebellum.Annu Rev Neurosci. 2018 Jul 8;41:233-253. doi: 10.1146/annurev-neuro-080317-061948. Annu Rev Neurosci. 2018. PMID: 29986160 Free PMC article. Review.
-
Differential Purkinje cell simple spike activity and pausing behavior related to cerebellar modules.J Neurophysiol. 2015 Apr 1;113(7):2524-36. doi: 10.1152/jn.00925.2014. Epub 2015 Feb 25. J Neurophysiol. 2015. PMID: 25717166 Free PMC article.
-
Purkinje Cell Representations of Behavior: Diary of a Busy Neuron.Neuroscientist. 2019 Jun;25(3):241-257. doi: 10.1177/1073858418785628. Epub 2018 Jul 9. Neuroscientist. 2019. PMID: 29985093 Free PMC article. Review.
-
Eye movements: the past 25 years.Vision Res. 2011 Jul 1;51(13):1457-83. doi: 10.1016/j.visres.2010.12.014. Epub 2011 Jan 13. Vision Res. 2011. PMID: 21237189 Free PMC article. Review.
References
-
- Albus JS. A theory of cerebellar function. Math Biosci 10: 25–61, 1971
-
- Buonomano DV, Mauk MD. Neural network model of the cerebellumml: temporal discrimination and the timing of motor responses. Neural Comput 6: 38–55, 1994
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

