Cytochrome c oxidase III as a mechanism for apoptosis in heart failure following myocardial infarction
- PMID: 19625613
- PMCID: PMC2770743
- DOI: 10.1152/ajpcell.00045.2009
Cytochrome c oxidase III as a mechanism for apoptosis in heart failure following myocardial infarction
Abstract
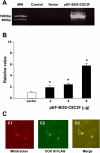
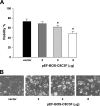
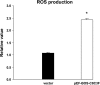
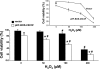
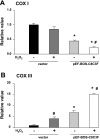
Cytochrome c oxidase (COX) is composed of 13 subunits, of which COX I, II, and III are encoded by a mitochondrial gene. COX I and II function as the main catalytic components, but the function of COX III is unclear. Because myocardial ischemia affects mitochondrial oxidative metabolism, we hypothesized that COX activity and expression would be affected during postischemic cardiomyopathy. This hypothesis was tested in a monkey model following myocardial infarction (MI) and subsequent pacing-induced heart failure (HF). In this model, COX I protein expression was decreased threefold after MI and fourfold after HF (P < 0.05 vs. sham), whereas COX II expression remained unchanged. COX III protein expression increased 5-fold after MI and further increased 10-fold after HF compared with sham (P < 0.05 vs. sham). The physiological impact of COX III regulation was examined in vitro. Overexpression of COX III in mitochondria of HL-1 cells resulted in an 80% decrease in COX I, 60% decrease in global COX activity, 60% decrease in cell viability, and threefold increase in apoptosis (P < 0.05). Oxidative stress induced by H2O2 significantly (P < 0.05) increased COX III expression. H2O2 decreased cell viability by 47 +/- 3% upon overexpression of COX III, but only by 12 +/- 5% in control conditions (P < 0.05). We conclude that ischemic stress in vivo and oxidative stress in vitro lead to upregulation of COX III, followed by downregulation of COX I expression, impaired COX oxidative activity, and increased apoptosis. Therefore, upregulation of COX III may contribute to the increased susceptibility to apoptosis following MI and subsequent HF.
Figures

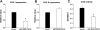

Similar articles
-
Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction.Circ Res. 2001 Mar 16;88(5):529-35. doi: 10.1161/01.res.88.5.529. Circ Res. 2001. PMID: 11249877
-
Proline improves cardiac remodeling following myocardial infarction and attenuates cardiomyocyte apoptosis via redox regulation.Biochem Pharmacol. 2020 Aug;178:114065. doi: 10.1016/j.bcp.2020.114065. Epub 2020 May 31. Biochem Pharmacol. 2020. PMID: 32492448
-
Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction.Free Radic Biol Med. 2007 Jul 15;43(2):271-81. doi: 10.1016/j.freeradbiomed.2007.04.021. Epub 2007 Apr 29. Free Radic Biol Med. 2007. PMID: 17603936
-
Mitochondrial ROS in myocardial ischemia reperfusion and remodeling.Biochim Biophys Acta Mol Basis Dis. 2020 Jul 1;1866(7):165768. doi: 10.1016/j.bbadis.2020.165768. Epub 2020 Mar 12. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32173461 Review.
-
Cytochrome c oxidase dysfunction in oxidative stress.Free Radic Biol Med. 2012 Sep 15;53(6):1252-63. doi: 10.1016/j.freeradbiomed.2012.07.021. Epub 2012 Jul 25. Free Radic Biol Med. 2012. PMID: 22841758 Free PMC article. Review.
Cited by
-
An Improved Monkey Model of Myocardial Ischemic Infarction for Cardiovascular Drug Development.Cardiovasc Toxicol. 2022 Sep;22(9):787-801. doi: 10.1007/s12012-022-09754-6. Epub 2022 Jun 23. Cardiovasc Toxicol. 2022. PMID: 35739384
-
The Mechanism Underlying the Protective Effects of Tannic Acid Against Isoproterenol-Induced Myocardial Fibrosis in Mice.Front Pharmacol. 2020 May 15;11:716. doi: 10.3389/fphar.2020.00716. eCollection 2020. Front Pharmacol. 2020. PMID: 32499705 Free PMC article.
-
Early markers for myocardial ischemia and sudden cardiac death.Int J Legal Med. 2016 Sep;130(5):1265-80. doi: 10.1007/s00414-016-1401-9. Epub 2016 Jul 8. Int J Legal Med. 2016. PMID: 27392959
-
Positron emission tomography imaging of cardiomyocyte apoptosis with a novel molecule probe [18F]FP-DPAZn2.Oncotarget. 2015 Oct 13;6(31):30579-91. doi: 10.18632/oncotarget.5769. Oncotarget. 2015. PMID: 26416423 Free PMC article.
-
Mitochondria and heart failure: new insights into an energetic problem.Minerva Cardioangiol. 2010 Apr;58(2):213-29. Minerva Cardioangiol. 2010. PMID: 20440251 Free PMC article. Review.
References
-
- Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr 31: 347–366, 1999 - PubMed
-
- Bauer M, Kristensen BW, Meyer M, Gasser T, Widmer HR, Zimmer J, Ueffing M. Toxic effects of lipid-mediated gene transfer in ventral mesencephalic explant cultures. Basic Clin Pharmacol Toxicol 98: 395–400, 2006 - PubMed
-
- Boveris A. Mitochondrial production of hydrogen peroxide in Saccharomyces cerevisiae. Acta Physiol Latinoam 26: 303–309, 1976 - PubMed
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 - PubMed
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

