Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes
- PMID: 19625617
- PMCID: PMC2722269
- DOI: 10.1073/pnas.0904636106
Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes
Erratum in
- Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16890. Photowala, Huzefa [added]
Abstract
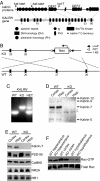
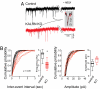
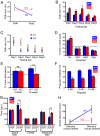
Dendritic spine morphogenesis contributes to brain function, cognition, and behavior, and is altered in psychiatric disorders. Kalirin is a brain-specific guanine-nucleotide exchange factor (GEF) for Rac-like GTPases and is a key regulator of spine morphogenesis. Here, we show that KALRN-knockout mice have specific reductions in cortical, but not hippocampal, Rac1 signaling and spine density, and exhibit reduced cortical glutamatergic transmission. These mice exhibit robust deficits in working memory, sociability, and prepulse inhibition, paralleled by locomotor hyperactivity reversible by clozapine in a kalirin-dependent manner. Several of these deficits are delayed and age-dependent. Our study thus links spine morphogenic signaling with age-dependent, delayed, disease-related phenotypes, including cognitive dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7.J Neurosci. 2008 Jun 11;28(24):6079-91. doi: 10.1523/JNEUROSCI.1170-08.2008. J Neurosci. 2008. PMID: 18550750 Free PMC article.
-
Hippocampal phenotypes in kalirin-deficient mice.Mol Cell Neurosci. 2011 Jan;46(1):45-54. doi: 10.1016/j.mcn.2010.08.005. Epub 2010 Aug 11. Mol Cell Neurosci. 2011. PMID: 20708080 Free PMC article.
-
Kalirin-9 and Kalirin-12 Play Essential Roles in Dendritic Outgrowth and Branching.Cereb Cortex. 2015 Oct;25(10):3487-501. doi: 10.1093/cercor/bhu182. Epub 2014 Aug 21. Cereb Cortex. 2015. PMID: 25146373 Free PMC article.
-
Kalirin signaling: implications for synaptic pathology.Mol Neurobiol. 2012 Feb;45(1):109-18. doi: 10.1007/s12035-011-8223-z. Epub 2011 Dec 23. Mol Neurobiol. 2012. PMID: 22194219 Free PMC article. Review.
-
Dendritic spine dynamics--a key role for kalirin-7.Trends Neurosci. 2008 Aug;31(8):419-27. doi: 10.1016/j.tins.2008.06.001. Epub 2008 Jul 1. Trends Neurosci. 2008. PMID: 18597863 Free PMC article. Review.
Cited by
-
The role of glial cells in mental illness: a systematic review on astroglia and microglia as potential players in schizophrenia and its cognitive and emotional aspects.Front Cell Neurosci. 2024 Feb 14;18:1358450. doi: 10.3389/fncel.2024.1358450. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38419655 Free PMC article.
-
Correlated evolution of social organization and lifespan in mammals.Nat Commun. 2023 Jan 31;14(1):372. doi: 10.1038/s41467-023-35869-7. Nat Commun. 2023. PMID: 36720880 Free PMC article.
-
Psychosis in Alzheimer's disease.Biol Psychiatry. 2014 Apr 1;75(7):542-52. doi: 10.1016/j.biopsych.2013.08.020. Epub 2013 Oct 6. Biol Psychiatry. 2014. PMID: 24103379 Free PMC article. Review.
-
Structural variants identified using non-Mendelian inheritance patterns advance the mechanistic understanding of autism spectrum disorder.HGG Adv. 2022 Oct 6;4(1):100150. doi: 10.1016/j.xhgg.2022.100150. eCollection 2023 Jan 12. HGG Adv. 2022. PMID: 36340933 Free PMC article.
-
Developmental Trajectories of Auditory Cortex Synaptic Structures and Gap-Prepulse Inhibition of Acoustic Startle Between Early Adolescence and Young Adulthood in Mice.Cereb Cortex. 2016 May;26(5):2115-26. doi: 10.1093/cercor/bhv040. Epub 2015 Mar 10. Cereb Cortex. 2016. PMID: 25759333 Free PMC article.
References
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Ann Rev Neurosci. 2007;30:79–97. - PubMed
-
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. - PubMed
-
- Penzes P, Cahill ME, Jones KA, Srivastava DP. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008;18:405–413. - PubMed
-
- Ma XM, Johnson RC, Mains RE, Eipper BA. Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. neurology. Comp Neurol. 2001;429:388–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 DA024761/DA/NIDA NIH HHS/United States
- P01 MH074866/MH/NIMH NIH HHS/United States
- P50 MH074866/MH/NIMH NIH HHS/United States
- R01MH071316/MH/NIMH NIH HHS/United States
- F31AG031621-01A2/AG/NIA NIH HHS/United States
- R01 AG017139/AG/NIA NIH HHS/United States
- R01 MH047340/MH/NIMH NIH HHS/United States
- MH57014/MH/NIMH NIH HHS/United States
- F31 AG031621/AG/NIA NIH HHS/United States
- R37 AG008796/AG/NIA NIH HHS/United States
- R37 NS034696/NS/NINDS NIH HHS/United States
- R37 AG08796/AG/NIA NIH HHS/United States
- R01 MH057014/MH/NIMH NIH HHS/United States
- R01 MH071316/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

