Role of human noncoding RNAs in the control of tumorigenesis
- PMID: 19625619
- PMCID: PMC2722347
- DOI: 10.1073/pnas.0906005106
Role of human noncoding RNAs in the control of tumorigenesis
Abstract
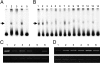
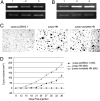
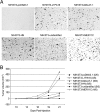
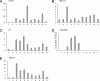
Related studies showed that the protein PSF represses proto-oncogene transcription, and VL30-1 RNA, a mouse noncoding retroelement RNA, binds and releases PSF from a proto-oncogene, activating transcription. Here we show that this mechanism regulates tumorigenesis in human cells, with human RNAs replacing VL30-1 RNA. A library of human RNA fragments was used to isolate, by affinity chromatography, 5 noncoding RNA fragments that bind to human PSF (hPSF), releasing hPSF from a proto-oncogene and activating transcription. Each of the 5 RNA fragments maps to a different human gene. The tumorigenic function of the hPSF-binding RNAs was tested in a human melanoma line and mouse fibroblast line, by determining the effect of the RNAs on formation of colonies in agar and tumors in mice. (i) Expressing in human melanoma cells the RNA fragments individually promoted tumorigenicity. (ii) Expressing in human melanoma cells a shRNA, which causes degradation of the endogenous RNA from which an RNA fragment was derived, suppressed tumorigenicity. (iii) Expressing in mouse NIH/3T3 cells the RNA fragments individually resulted in transformation to tumorigenic cells. (iv) A screen of 9 human tumor lines showed that each line expresses high levels of several hPSF-binding RNAs, relative to the levels in human fibroblast cells. We conclude that human hPSF-binding RNAs drive transformation and tumorigenesis by reversing PSF-mediated repression of proto-oncogene transcription and that dysfunctional regulation of human hPSF-binding RNA expression has a central role in the etiology of human cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Regulation of proto-oncogene transcription, cell proliferation, and tumorigenesis in mice by PSF protein and a VL30 noncoding RNA.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16794-8. doi: 10.1073/pnas.0909022106. Epub 2009 Sep 11. Proc Natl Acad Sci U S A. 2009. PMID: 19805375 Free PMC article.
-
Roles of PSF protein and VL30 RNA in reversible gene regulation.Proc Natl Acad Sci U S A. 2005 Aug 23;102(34):12189-93. doi: 10.1073/pnas.0505179102. Epub 2005 Aug 3. Proc Natl Acad Sci U S A. 2005. PMID: 16079199 Free PMC article.
-
Binding of mouse VL30 retrotransposon RNA to PSF protein induces genes repressed by PSF: effects on steroidogenesis and oncogenesis.Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):621-6. doi: 10.1073/pnas.0307794100. Epub 2004 Jan 2. Proc Natl Acad Sci U S A. 2004. PMID: 14704271 Free PMC article.
-
From a retrovirus infection of mice to a long noncoding RNA that induces proto-oncogene transcription and oncogenesis via an epigenetic transcription switch.Signal Transduct Target Ther. 2016 May 13;1:16007. doi: 10.1038/sigtrans.2016.7. eCollection 2016. Signal Transduct Target Ther. 2016. PMID: 29263895 Free PMC article. Review.
-
Hacking RNA: Hakai promotes tumorigenesis by enhancing the RNA-binding function of PSF.Cell Cycle. 2009 Nov 15;8(22):3648-51. doi: 10.4161/cc.8.22.9909. Epub 2009 Nov 24. Cell Cycle. 2009. PMID: 19855157 Free PMC article. Review.
Cited by
-
Uncovering the role of genomic "dark matter" in human disease.J Clin Invest. 2012 May;122(5):1589-95. doi: 10.1172/JCI60020. Epub 2012 May 1. J Clin Invest. 2012. PMID: 22546862 Free PMC article.
-
Downregulation of MALAT1 is a hallmark of tissue and peripheral proliferative T cells in COVID-19.Clin Exp Immunol. 2023 Jun 5;212(3):262-275. doi: 10.1093/cei/uxad034. Clin Exp Immunol. 2023. PMID: 36869729 Free PMC article.
-
Association between long non-coding RNA polymorphisms and cancer risk: a meta-analysis.Biosci Rep. 2018 Jul 31;38(4):BSR20180365. doi: 10.1042/BSR20180365. Print 2018 Aug 31. Biosci Rep. 2018. PMID: 29802154 Free PMC article.
-
The long noncoding RNA HOXA transcript at the distal tip promotes colorectal cancer growth partially via silencing of p21 expression.Tumour Biol. 2016 Jun;37(6):7431-40. doi: 10.1007/s13277-015-4617-2. Epub 2015 Dec 17. Tumour Biol. 2016. PMID: 26678886
-
Distinct lncRNA transcriptional fingerprints characterize progressive stages of multiple myeloma.Oncotarget. 2016 Mar 22;7(12):14814-30. doi: 10.18632/oncotarget.7442. Oncotarget. 2016. PMID: 26895470 Free PMC article.
References
-
- Patton JG, Porro EB, Galceran J, Tempst P, Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Develop. 1993;7:393–406. - PubMed
-
- Garen A, Song X. Regulatory roles of tumor-suppressor proteins and noncoding RNAs in cancer and normal cell functions. Int J Cancer. 2008;122:1687–1689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

