Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the allosteric properties of the enzyme
- PMID: 19625637
- PMCID: PMC2735977
- DOI: 10.1104/pp.109.138933
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the allosteric properties of the enzyme
Abstract
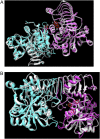
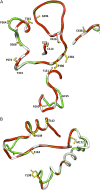
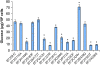
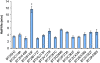
ADP-glucose pyrophosphorylase (AGPase) catalyzes a rate-limiting step in glycogen and starch synthesis in bacteria and plants, respectively. Plant AGPase consists of two large and two small subunits that were derived by gene duplication. AGPase large subunits have functionally diverged, leading to different kinetic and allosteric properties. Amino acid changes that could account for these differences were identified previously by evolutionary analysis. In this study, these large subunit residues were mapped onto a modeled structure of the maize (Zea mays) endosperm enzyme. Surprisingly, of 29 amino acids identified via evolutionary considerations, 17 were located at subunit interfaces. Fourteen of the 29 amino acids were mutagenized in the maize endosperm large subunit (SHRUNKEN-2 [SH2]), and resulting variants were expressed in Escherichia coli with the maize endosperm small subunit (BT2). Comparisons of the amount of glycogen produced in E. coli, and the kinetic and allosteric properties of the variants with wild-type SH2/BT2, indicate that 11 variants differ from the wild type in enzyme properties or in vivo glycogen level. More interestingly, six of nine residues located at subunit interfaces exhibit altered allosteric properties. These results indicate that the interfaces between the large and small subunits are important for the allosteric properties of AGPase, and changes at these interfaces contribute to AGPase functional specialization. Our results also demonstrate that evolutionary analysis can greatly facilitate enzyme structure-function analyses.
Figures



Similar articles
-
Enhanced heat stability and kinetic parameters of maize endosperm ADPglucose pyrophosphorylase by alteration of phylogenetically identified amino acids.Arch Biochem Biophys. 2014 Feb 1;543:1-9. doi: 10.1016/j.abb.2013.12.018. Epub 2013 Dec 27. Arch Biochem Biophys. 2014. PMID: 24378757
-
Characterization of an autonomously activated plant ADP-glucose pyrophosphorylase.Plant Physiol. 2009 Jan;149(1):318-26. doi: 10.1104/pp.108.126862. Epub 2008 Aug 20. Plant Physiol. 2009. PMID: 18715954 Free PMC article.
-
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase.Plant Physiol. 2010 Jan;152(1):85-95. doi: 10.1104/pp.109.146928. Epub 2009 Nov 4. Plant Physiol. 2010. PMID: 19889875 Free PMC article.
-
Structure, function, and evolution of plant ADP-glucose pyrophosphorylase.Plant Mol Biol. 2022 Mar;108(4-5):307-323. doi: 10.1007/s11103-021-01235-8. Epub 2022 Jan 10. Plant Mol Biol. 2022. PMID: 35006475 Review.
-
The evolution of the starch biosynthetic pathway in cereals and other grasses.J Exp Bot. 2009;60(9):2481-92. doi: 10.1093/jxb/erp141. J Exp Bot. 2009. PMID: 19505928 Review.
Cited by
-
A brittle-2 transgene increases maize yield by acting in maternal tissues to increase seed number.Plant Direct. 2017 Dec 7;1(6):e00029. doi: 10.1002/pld3.29. eCollection 2017 Dec. Plant Direct. 2017. PMID: 31245677 Free PMC article.
-
Contrasted patterns of selection since maize domestication on duplicated genes encoding a starch pathway enzyme.Theor Appl Genet. 2011 Mar;122(4):705-22. doi: 10.1007/s00122-010-1480-9. Theor Appl Genet. 2011. PMID: 21060986
-
Lineage-Specific Evolutionary Histories and Regulation of Major Starch Metabolism Genes during Banana Ripening.Front Plant Sci. 2016 Dec 2;7:1778. doi: 10.3389/fpls.2016.01778. eCollection 2016. Front Plant Sci. 2016. PMID: 27994606 Free PMC article.
-
A critical inter-subunit interaction for the transmission of the allosteric signal in the Agrobacterium tumefaciens ADP-glucose pyrophosphorylase.Protein Sci. 2023 Sep;32(9):e4747. doi: 10.1002/pro.4747. Protein Sci. 2023. PMID: 37551561 Free PMC article.
-
Structural comparison, substrate specificity, and inhibitor binding of AGPase small subunit from monocot and dicot: present insight and future potential.Biomed Res Int. 2014;2014:583606. doi: 10.1155/2014/583606. Epub 2014 Sep 2. Biomed Res Int. 2014. PMID: 25276800 Free PMC article.
References
-
- Akihiro T, Mizuno K, Fujimura T (2005) Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol 46 937–946 - PubMed
-
- Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL Workspace: a Web-based environment for protein structure homology modeling. Bioinformatics 22 195–201 - PubMed
-
- Ballicora MA, Dubay JR, Devillers CH, Preiss J (2005) Resurrecting the ancestral enzymatic role of a modulatory subunit. J Biol Chem 280 10189–10195 - PubMed
-
- Ballicora MA, Erben ED, Yazaki T, Bertolo AL, Demonte AM, Schmidt JR, Aleanzi M, Bejar CM, Figueroa CM, Fusari CM, et al (2007) Identification of regions critically affecting kinetics and allosteric regulation of the Escherichia coli ADP-glucose pyrophosphorylase by modeling and pentapeptide-scanning mutagenesis. J Bacteriol 189 5325–5333 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

