Human neutrophils coordinate chemotaxis by differential activation of Rac1 and Rac2
- PMID: 19625648
- PMCID: PMC3056163
- DOI: 10.4049/jimmunol.0900849
Human neutrophils coordinate chemotaxis by differential activation of Rac1 and Rac2
Abstract
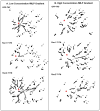
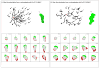
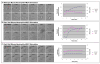
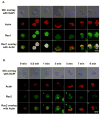
Rac1 and Rac2, members of the small Rho GTPase family, play essential roles in coordinating directional migration and superoxide production during neutrophil responses to chemoattractants. Although earlier studies in Rac1 and Rac2 knockout mice have demonstrated unique roles for each Rac isoform in chemotaxis and NADPH oxidase activation, it is still unclear how human neutrophils use Rac1 and Rac2 to achieve their immunological responses to foreign agent stimulation. In the current study, we used TAT dominant-negative Rac1-T17N and Rac2-T17N fusion proteins to acutely alter the activity of Rac1 and Rac2 individually in human neutrophils. We demonstrate distinct activation kinetics and different roles for Rac1 and Rac2 in response to low vs high concentrations of fMLP. These observations were verified using neutrophils from mice in which Rac1 or Rac2 was genetically absent. Based on these results, we propose a model to explain how human neutrophils kill invading microbes while limiting oxidative damage to the adjacent surrounding healthy tissue through the differential activation of Rac1 and Rac2 in response to different concentrations of chemoattractant.
Figures



Similar articles
-
Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions.J Immunol. 2002 Nov 1;169(9):5043-51. doi: 10.4049/jimmunol.169.9.5043. J Immunol. 2002. PMID: 12391220
-
Rac GTPase isoform-specific regulation of NADPH oxidase and chemotaxis in murine neutrophils in vivo. Role of the C-terminal polybasic domain.J Biol Chem. 2005 Jan 14;280(2):953-64. doi: 10.1074/jbc.M408820200. Epub 2004 Oct 25. J Biol Chem. 2005. PMID: 15504745
-
Impaired NADPH oxidase activity in Rac2-deficient murine neutrophils does not result from defective translocation of p47phox and p67phox and can be rescued by exogenous arachidonic acid.J Leukoc Biol. 2006 Jan;79(1):223-34. doi: 10.1189/jlb.0705371. Epub 2005 Nov 7. J Leukoc Biol. 2006. PMID: 16275890
-
Rac GTPases in human diseases.Dis Markers. 2010;29(3-4):177-87. doi: 10.3233/DMA-2010-0738. Dis Markers. 2010. PMID: 21178276 Free PMC article. Review.
-
Regulation of neutrophil function by Rac GTPases.Curr Opin Hematol. 2003 Jan;10(1):8-15. doi: 10.1097/00062752-200301000-00003. Curr Opin Hematol. 2003. PMID: 12483106 Review.
Cited by
-
Agent-based modeling approach of immune defense against spores of opportunistic human pathogenic fungi.Front Microbiol. 2012 Apr 26;3:129. doi: 10.3389/fmicb.2012.00129. eCollection 2012. Front Microbiol. 2012. PMID: 22557995 Free PMC article.
-
Proteomic analysis of secretagogue-stimulated neutrophils implicates a role for actin and actin-interacting proteins in Rac2-mediated granule exocytosis.Proteome Sci. 2011 Nov 14;9:70. doi: 10.1186/1477-5956-9-70. Proteome Sci. 2011. PMID: 22081935 Free PMC article.
-
Optical Tools To Study the Isoform-Specific Roles of Small GTPases in Immune Cells.J Immunol. 2016 Apr 15;196(8):3479-93. doi: 10.4049/jimmunol.1501655. Epub 2016 Mar 7. J Immunol. 2016. PMID: 26951800 Free PMC article.
-
Single nucleotide polymorphisms that increase expression of the guanosine triphosphatase RAC1 are associated with ulcerative colitis.Gastroenterology. 2011 Aug;141(2):633-41. doi: 10.1053/j.gastro.2011.04.057. Epub 2011 May 4. Gastroenterology. 2011. PMID: 21684284 Free PMC article.
-
Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue.Dev Cell. 2011 Oct 18;21(4):735-45. doi: 10.1016/j.devcel.2011.07.013. Dev Cell. 2011. PMID: 22014524 Free PMC article.
References
-
- Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz) 2005;53:505–517. - PubMed
-
- Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. - PubMed
-
- Fenteany G, Glogauer M. Cytoskeletal remodeling in leukocyte function. Curr Opin Hematol. 2004;11:15–24. - PubMed
-
- Bokoch GM, Zhao T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533–1548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

