Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells
- PMID: 19625659
- PMCID: PMC2775007
- DOI: 10.1096/fj.09-134882
Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells
Abstract
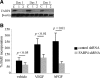
Fatty acid binding protein 4 (FABP4) plays an important role in maintaining glucose and lipid homeostasis. FABP4 has been primarily regarded as an adipocyte- and macrophage-specific protein, but recent studies suggest that it may be more widely expressed. We found strong FABP4 expression in the endothelial cells (ECs) of capillaries and small veins in several mouse and human tissues, including the heart and kidney. FABP4 was also detected in the ECs of mature human placental vessels and infantile hemangiomas, the most common tumor of infancy and ECs. In most of these cases, FABP4 was detected in both the nucleus and cytoplasm. FABP4 mRNA and protein levels were significantly induced in cultured ECs by VEGF-A and bFGF treatment. The effect of VEGF-A on FABP4 expression was inhibited by chemical inhibition or short-hairpin (sh) RNA-mediated knockdown of VEGF-receptor-2 (R2), whereas the VEGFR1 agonists, placental growth factors 1 and 2, had no effect on FABP4 expression. Knockdown of FABP4 in ECs significantly reduced proliferation both under baseline conditions and in response to VEGF and bFGF. Thus, FABP4 emerged as a novel target of the VEGF/VEGFR2 pathway and a positive regulator of cell proliferation in ECs.
Figures




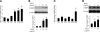
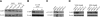
References
-
- Hertzel A V, Bernlohr D A. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11:175–180. - PubMed
-
- Haunerland N H, Spener F. Fatty acid-binding proteins—insights from genetic manipulations. Prog Lipid Res. 2004;43:328–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

