Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children
- PMID: 19625687
- PMCID: PMC3136010
- DOI: 10.3945/ajcn.2009.27462AA
Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children
Abstract
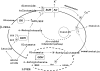
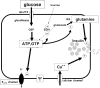
In addition to its extracellular roles as a neurotransmitter/sensory molecule, glutamate serves important intracellular signaling functions via its metabolism through glutamate dehydrogenase (GDH). GDH is a mitochondrial matrix enzyme that catalyzes the oxidative deamination of glutamate to alpha-ketoglutarate in a limited number of tissues in humans, including the liver, the kidney, the brain, and the pancreatic islets. GDH activity is subject to complex regulation by negative (GTP, palmitoyl-coenzyme A) and positive (ADP, leucine) allosteric effectors. This complex regulation allows GDH activity to be modulated by changes in energy state and amino acid availability. The importance of GDH regulation has been highlighted by the discovery of a novel hypoglycemic disorder in children, the hyperinsulinism-hyperammonemia syndrome, which is caused by dominantly expressed, activating mutations of the enzyme that impair its inhibition by GTP. Affected children present in infancy with hypoglycemic seizures after brief periods of fasting or the ingestion of a high-protein meal. Patients have characteristic persistent 3- to 5-fold elevations of blood ammonia concentrations but do not display the usual neurologic symptoms of hyperammonemia. The mutant GDH enzyme shows impaired responses to GTP inhibition. Isolated islets from mice that express the mutant GDH in pancreatic beta cells show an increased rate of glutaminolysis, increased insulin release in response to glutamine, and increased sensitivity to leucine-stimulated insulin secretion. The novel hyperinsulinism-hyperammonemia syndrome indicates that GDH-catalyzed glutamate metabolism plays important roles in 3 tissues: in beta cells, the regulation of amino acid-stimulated insulin secretion; in hepatocytes, the modulation of amino acid catabolism and ammoniagenesis; and in brain neurons, the maintenance of glutamate neurotransmitter concentrations.
Figures


References
-
- MacMullen C, Fang J, Hsu BYL, et al. Hyperinsulinism/hyperammonemia syndrome in children with regulatory mutations in the inhibitory GTP binding domain of glutamate dehydrogenase. J Clin Endocrinol Metab 2001;86:1782–7 - PubMed
-
- Stanley CA, Lieu YK, Hsu BY, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 1998;338:1352–7 - PubMed
-
- Smith TJ, Peterson PE, Schmidt T, Fang J, Stanley CA. Structures of bovine glutamate dehydrogenase complexes elucidate the mechanism of purine regulation. J Mol Biol 2001;307:707–20 - PubMed
-
- Allen A, Kwagh J, Fang J, Stanley CA, Smith TJ. Evolution of glutamate dehydrogenase regulation of insulin homeostasis is an example of molecular exaptation. Biochemistry 2004;43:14431–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

