Sustained stimulation of vasopressin and oxytocin release by ATP and phenylephrine requires recruitment of desensitization-resistant P2X purinergic receptors
- PMID: 19625689
- PMCID: PMC2763823
- DOI: 10.1152/ajpregu.00358.2009
Sustained stimulation of vasopressin and oxytocin release by ATP and phenylephrine requires recruitment of desensitization-resistant P2X purinergic receptors
Abstract
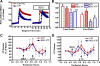
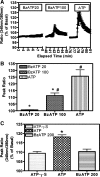
Coexposure of hypothalamo-neurohypophyseal system explants to ATP and phenylephrine [PE; an alpha1-adrenergic receptor (alpha1-AR) agonist] induces an extended elevation in vasopressin and oxytocin (VP/OT) release. New evidence is presented that this extended response is mediated by recruitment of desensitization-resistant ionotropic purinergic receptor subtypes (P2X-Rs): 1) Antagonists of the P2X2/3 and P2X7-Rs truncated the sustained VP/OT release induced by ATP+PE but did not alter the transient response to ATP alone. 2) The P2X2/3 and P2X7-R antagonists did not alter either ATP or ATP+PE-induced increases in [Ca(2+)](i). 3) P2X2/3 and P2X7-R agonists failed to elevate [Ca(2+)](i), while ATP-gamma-S, an agonist for P2X2-Rs increased [Ca(2+)](i) and induced a transient increase in VP/OT release. 4) A P2Y1-R antagonist did not prevent initiation of the synergistic, sustained stimulation of VP/OT release by ATP+PE but did reduce its duration. Thus, the desensitization-resistant P2X2/3 and P2X7-R subtypes are required for the sustained, synergistic hormone response to ATP+PE, while P2X2-Rs are responsible for the initial activation of Ca(2+)-influx by ATP and ATP stimulation of VP/OT release. Immunohistochemistry, coimmunoprecipitation, and Western blot analysis confirmed the presence of P2X2 and P2X3, P2X2/3, and P2X7-R protein, respectively in SON. These findings support the hypothesis that concurrent activation of P2X2-R and alpha1-AR induces calcium-driven recruitment of P2X2/3 and 7-Rs, allowing sustained activation of a homeostatic circuit. Recruitment of these receptors may provide sustained release of VP during dehydration and may be important for preventing hemorrhagic and septic shock.
Figures







Similar articles
-
Multiple alpha1-adrenergic receptor subtypes support synergistic stimulation of vasopressin and oxytocin release by ATP and phenylephrine.Am J Physiol Regul Integr Comp Physiol. 2010 Dec;299(6):R1529-37. doi: 10.1152/ajpregu.00532.2010. Epub 2010 Sep 29. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20881098 Free PMC article.
-
Role of purinergic P2Y1 receptors in regulation of vasopressin and oxytocin secretion.Am J Physiol Regul Integr Comp Physiol. 2009 Aug;297(2):R478-84. doi: 10.1152/ajpregu.00163.2009. Epub 2009 Jun 10. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19515986
-
Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release.J Neurosci. 2000 Dec 1;20(23):8868-75. doi: 10.1523/JNEUROSCI.20-23-08868.2000. J Neurosci. 2000. PMID: 11102496 Free PMC article.
-
Regulation of vasopressin release by co-released neurotransmitters: mechanisms of purinergic and adrenergic synergism.Prog Brain Res. 2008;170:93-107. doi: 10.1016/S0079-6123(08)00409-3. Prog Brain Res. 2008. PMID: 18655875 Review.
-
Pharmacology of cloned P2X receptors.Annu Rev Pharmacol Toxicol. 2000;40:563-80. doi: 10.1146/annurev.pharmtox.40.1.563. Annu Rev Pharmacol Toxicol. 2000. PMID: 10836147 Review.
Cited by
-
P2X2 Receptor Expression and Function Is Upregulated in the Rat Supraoptic Nucleus Stimulated Through Refeeding After Fasting.Front Cell Neurosci. 2019 Jun 26;13:284. doi: 10.3389/fncel.2019.00284. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31297050 Free PMC article.
-
Purinergic signalling in endocrine organs.Purinergic Signal. 2014 Mar;10(1):189-231. doi: 10.1007/s11302-013-9396-x. Epub 2013 Nov 22. Purinergic Signal. 2014. PMID: 24265070 Free PMC article. Review.
-
Extracellular ATP and other nucleotides-ubiquitous triggers of intercellular messenger release.Purinergic Signal. 2016 Mar;12(1):25-57. doi: 10.1007/s11302-015-9483-2. Epub 2015 Nov 6. Purinergic Signal. 2016. PMID: 26545760 Free PMC article. Review.
-
Function of Connexin-43 in Macrophages.Int J Mol Sci. 2021 Jan 30;22(3):1412. doi: 10.3390/ijms22031412. Int J Mol Sci. 2021. PMID: 33573367 Free PMC article. Review.
-
P2X7 receptors in neurohypophysial terminals: evidence for their role in arginine-vasopressin secretion.J Cell Physiol. 2014 Mar;229(3):333-42. doi: 10.1002/jcp.24453. J Cell Physiol. 2014. PMID: 24037803 Free PMC article.
References
-
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84, 2003 - PubMed
-
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol 376: 127–138, 1999 - PubMed
-
- Blair ML, Mickelsen D. Plasma protein and blood volume restitution after hemorrhage in conscious pregnant and ovarian steroid-replaced rats. Am J Physiol Regul Integr Comp Physiol 290: R425–R434, 2006 - PubMed
-
- Boue-Grabot E, Archambault V, Seguela P. A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X2 ATP-gated channels. J Biol Chem 275: 10190–10195, 2000 - PubMed
-
- Buell G, Michel AD, Lewis C, Collo G, Humphrey PP, Surprenant A. P2X1 receptor activation in HL60 cells. Blood 87: 2659–2664, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

