Chronic ethanol intake alters circadian phase shifting and free-running period in mice
- PMID: 19625732
- PMCID: PMC3049296
- DOI: 10.1177/0748730409338449
Chronic ethanol intake alters circadian phase shifting and free-running period in mice
Abstract
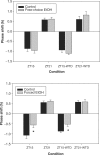
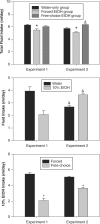
Chronic alcohol intake is associated with widespread disruptions in sleep and circadian rhythms in both human alcoholics and in experimental animals. Recent studies have demonstrated that chronic and acute ethanol treatments alter fundamental properties of the circadian pacemaker--including free-running period and responsiveness to photic and nonphotic phase-shifting stimuli--in rats and hamsters. In the present work, the authors extend these observations to the C57BL/6J mouse, an inbred strain characterized by very high levels of voluntary ethanol intake and by reliable and stable free-running circadian activity rhythms. Mice were housed individually in running-wheel cages under conditions of either voluntary or forced ethanol intake, whereas controls were maintained on plain water. Forced ethanol intake significantly attenuated photic phase delays (but not phase advances) and shortened free-running period in constant darkness, but voluntary ethanol intake failed to affect either of these parameters. Thus, high levels of chronic ethanol intake, beyond those normally achieved under voluntary drinking conditions, are required to alter fundamental circadian pacemaker properties in C57BL/6J mice. These observations may be related to the relative ethanol insensitivity displayed by this strain in several other phenotypic domains, including ethanol-induced sedation, ataxia, and withdrawal. Additional experiments will investigate chronobiological sensitivity to ethanol in a range of inbred strains showing diverse ethanol-related phenotypes.
Figures



Similar articles
-
Chronobiology of alcohol: studies in C57BL/6J and DBA/2J inbred mice.Physiol Behav. 2013 Feb 17;110-111:140-7. doi: 10.1016/j.physbeh.2013.01.001. Epub 2013 Jan 10. Physiol Behav. 2013. PMID: 23313401 Free PMC article.
-
Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster.Pharmacol Biochem Behav. 2007 Aug-Sep;87(3):297-305. doi: 10.1016/j.pbb.2007.05.001. Epub 2007 May 10. Pharmacol Biochem Behav. 2007. PMID: 17544066 Free PMC article.
-
Chronic ethanol intake alters circadian period-responses to brief light pulses in rats.Chronobiol Int. 2005;22(2):227-36. doi: 10.1081/cbi-200053496. Chronobiol Int. 2005. PMID: 16021840
-
[Aspects of chronobiological effects of ethanol].Psychiatr Pol. 2003 May-Jun;37(3):503-10. Psychiatr Pol. 2003. PMID: 13677979 Review. Polish.
-
Chronobiology of ethanol: animal models.Alcohol. 2015 Jun;49(4):311-9. doi: 10.1016/j.alcohol.2015.04.001. Epub 2015 Apr 21. Alcohol. 2015. PMID: 25971539 Review.
Cited by
-
Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability.Am J Physiol Gastrointest Liver Physiol. 2013 Jul 15;305(2):G185-95. doi: 10.1152/ajpgi.00354.2012. Epub 2013 May 9. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23660503 Free PMC article.
-
How Psychoactive Drugs and the Circadian Clock Are Enlightening One Another.Adv Exp Med Biol. 2021;1344:129-152. doi: 10.1007/978-3-030-81147-1_8. Adv Exp Med Biol. 2021. PMID: 34773230
-
Differential Sensitivity to Ethanol-Induced Circadian Rhythm Disruption in Adolescent and Adult Mice.Alcohol Clin Exp Res. 2017 Jan;41(1):187-196. doi: 10.1111/acer.13275. Epub 2016 Dec 20. Alcohol Clin Exp Res. 2017. PMID: 27997028 Free PMC article.
-
Selective breeding for ethanol-related traits alters circadian phenotype.Alcohol. 2013 May;47(3):187-94. doi: 10.1016/j.alcohol.2013.01.001. Epub 2013 Feb 13. Alcohol. 2013. PMID: 23414725 Free PMC article.
-
Circadian wheel-running activity during withdrawal from chronic intermittent ethanol exposure in mice.Alcohol. 2010 May;44(3):239-44. doi: 10.1016/j.alcohol.2010.02.011. Alcohol. 2010. PMID: 20682191 Free PMC article.
References
-
- Araujo NP, Camarini R, Souza-Formigoni ML, Carvalho RC, Abilio VC, Silva RH, Ricardo VP, Ribeiro Rde A, Frussa-Filho R. The importance of housing conditions on behavioral sensitization and tolerance to ethanol. Pharmacol Biochem Behav. 2005;82:40–45s. - PubMed
-
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. - PubMed
-
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. - PubMed
-
- Burke LP, Kramer SZ. Effects of photoperiod, melatonin and pinealectomy on ethanol consumption in rats. Pharmacol Biochem Behav. 1974;2:459–463. - PubMed
-
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

