The cardiac alpha(1C) subunit can support excitation-triggered Ca2+ entry in dysgenic and dyspedic myotubes
- PMID: 19625771
- PMCID: PMC2927111
- DOI: 10.4161/chan.3.4.9342
The cardiac alpha(1C) subunit can support excitation-triggered Ca2+ entry in dysgenic and dyspedic myotubes
Abstract
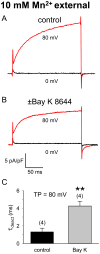
Depolarization-induced entry of divalent ions into skeletal muscle has been attributed to a process termed Excitation-Coupled Ca(2+) Entry (ECCE), which is hypothesized to require the interaction of the ryanodine receptor (RyR1), the L-type Ca(2+) channel (DHPR) and another unidentified cation channel. Thus, ECCE is absent in myotubes lacking either the DHPR (dysgenic) or RyR1 (dyspedic). Furthermore, ECCE, as measured by Mn(2+) quench of Fura-2, is reconstituted by expression of a mutant DHPR alpha(1S) subunit (SkEIIIK) thought to be impermeable to divalent cations. Previously, we showed that the bulk of depolarization-induced Ca(2+) entry could be explained by the skeletal L-type current. Accordingly, one would predict that any Ca(2+) current similar to the endogenous current would restore such entry and that this entry would not require coupling to either the DHPR or RyR1. Here, we show that expression of the cardiac alpha(1C) subunit in either dysgenic or dyspedic myotubes does result in Ca(2+) entry similar to that ascribed to ECCE. We also demonstrate that, when potentiated by strong depolarization and Bay K 8644, SkEIIIK supports entry of Mn(2+). These results strongly support the idea that the L-type channel is the major route of Ca(2+) entry in response to repetitive or prolonged depolarization of skeletal muscle.
Figures



References
-
- Hurne AM, O’Brien JJ, Wingrove D, Cherednichenko G, Allen PD, Beam KG, Pessah IN. Ryanodine receptor type I (RyR1) mutations C4958S and C4961S reveal excitation-coupled calcium entry (ECCE) is independent of sarcoplasmic reticulum store depletion. J Biol Chem. 2005;280:36994–7004. - PubMed
-
- Yang T, Allen PD, Pessah IN, López JR. Enhanced excitation-coupled calcium entry in myotubes is associated with expression of RyR1 malignant hyperthermia mutations. J Biol Chem. 2007;282:37471–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
