Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression
- PMID: 19625798
- PMCID: PMC2739411
- DOI: 10.1097/ALN.0b013e3181ae63d1
Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression
Abstract
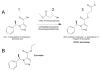
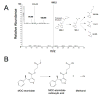
Background: Etomidate is a rapidly acting sedative-hypnotic that provides hemodynamic stability. It causes prolonged suppression of adrenocortical steroid synthesis; therefore, its clinical utility and safety are limited. The authors describe the results of studies to define the pharmacology of (R)-3-methoxy-3-oxopropyl1-(1-phenylethyl)-1H-imidazole-5-carboxylate (MOC-etomidate), the first etomidate analogue designed to be susceptible to ultra-rapid metabolism.
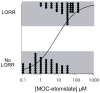
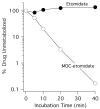
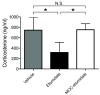
Methods: The gamma-aminobutyric acid type A receptor activities of MOC-etomidate and etomidate were compared by using electrophysiological techniques in human alpha1beta2gamma2l receptors. MOC-etomidate's hypnotic concentration was determined in tadpoles by using a loss of righting reflex assay. Its in vitro metabolic half-life was measured in human liver S9 fraction, and the resulting metabolite was provisionally identified by using high-performance liquid chromatography/mass spectrometry techniques. The hypnotic and hemodynamic actions of MOC-etomidate, etomidate, and propofol were defined in rats. The abilities of MOC-etomidate and etomidate to inhibit corticosterone production were assessed in rats.
Results: MOC-etomidate potently enhanced gamma-aminobutyric acid type A receptor function and produced loss of righting reflex in tadpoles. Metabolism in human liver S9 fraction was first-order, with an in vitro half-life of 4.4 min versus more than 40 min for etomidate. MOC-etomidate's only detectable metabolite was a carboxylic acid. In rats, MOC-etomidate produced rapid loss of righting reflex that was extremely brief and caused minimal hemodynamic changes. Unlike etomidate, MOC-etomidate produced no adrenocortical suppression 30 min after administration.
Conclusions: MOC-etomidate is an etomidate analogue that retains etomidate's important favorable pharmacological properties. However, it is rapidly metabolized, ultra-short-acting, and does not produce prolonged adrenocortical suppression after bolus administration.
Conflict of interest statement
Conflict of Interest Statement: The Massachusetts General Hospital has submitted a patent application for MOC-etomidate and related analogues. Five authors (Raines, Cotten, Forman, Husain, and Miller), their laboratories, the Dept. of Anesthesia and Critical Care at the Massachusetts General Hospital, and the Massachusetts General Hospital could receive royalties relating to the development or sale of these drugs.
Figures
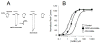
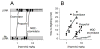

Comment in
-
Is anesthesiology going soft?: trends in fragile pharmacology.Anesthesiology. 2009 Aug;111(2):229-30. doi: 10.1097/ALN.0b013e3181ae8460. Anesthesiology. 2009. PMID: 19625797 No abstract available.
References
-
- de Ruiter G, Popescu DT, de Boer AG, Smeekens JB, Breimer DD. Pharmacokinetics of etomidate in surgical patients. Arch Int Pharmacodyn Ther. 1981;249:180–8. - PubMed
-
- Hebron BS, Edbrooke DL, Newby DM, Mather SJ. Pharmacokinetics of etomidate associated with prolonged i.v. infusion. Br J Anaesth. 1983;55:281–7. - PubMed
-
- McCollum JS, Dundee JW. Comparison of induction characteristics of four intravenous anaesthetic agents. Anaesthesia. 1986;41:995–1000. - PubMed
-
- Gooding JM, Weng JT, Smith RA, Berninger GT, Kirby RR. Cardiovascular and pulmonary responses following etomidate induction of anesthesia in patients with demonstrated cardiac disease. Anesth Analg. 1979;58:40–1. - PubMed
-
- Gooding JM, Corssen G. Effect of etomidate on the cardiovascular system. Anesth Analg. 1977;56:717–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

