XIAP discriminates between type I and type II FAS-induced apoptosis
- PMID: 19626005
- PMCID: PMC2956120
- DOI: 10.1038/nature08229
XIAP discriminates between type I and type II FAS-induced apoptosis
Abstract
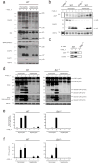
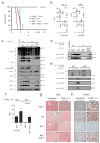
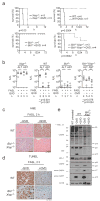
FAS (also called APO-1 and CD95) and its physiological ligand, FASL, regulate apoptosis of unwanted or dangerous cells, functioning as a guardian against autoimmunity and cancer development. Distinct cell types differ in the mechanisms by which the 'death receptor' FAS triggers their apoptosis. In type I cells, such as lymphocytes, activation of 'effector caspases' by FAS-induced activation of caspase-8 suffices for cell killing, whereas in type II cells, including hepatocytes and pancreatic beta-cells, caspase cascade amplification through caspase-8-mediated activation of the pro-apoptotic BCL-2 family member BID (BH3 interacting domain death agonist) is essential. Here we show that loss of XIAP (X-chromosome linked inhibitor of apoptosis protein) function by gene targeting or treatment with a second mitochondria-derived activator of caspases (SMAC, also called DIABLO; direct IAP-binding protein with low pI) mimetic drug in mice rendered hepatocytes and beta-cells independent of BID for FAS-induced apoptosis. These results show that XIAP is the critical discriminator between type I and type II apoptosis signalling and suggest that IAP inhibitors should be used with caution in cancer patients with underlying liver conditions.
Figures

Comment in
-
Shortcut to death.Hepatology. 2009 Dec;50(6):2040-3. doi: 10.1002/hep.23400. Hepatology. 2009. PMID: 19937700 No abstract available.
References
-
- Nagata S. Fas ligand-induced apoptosis. Annual Review of Genetics. 1999;33:29–55. - PubMed
-
- Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. - PubMed
-
- Peter ME, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. - PubMed
-
- Wang K, Yin X-M, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes and Development. 1996;10:2859–2869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

