General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats
- PMID: 19626389
- PMCID: PMC3629551
- DOI: 10.1007/s12640-009-9088-z
General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats
Abstract
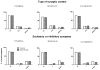
Common general anesthetics administered to young rats at the peak of brain development cause widespread apoptotic neurodegeneration in their immature brain. Behavioral studies have shown that this leads to learning and memory deficiencies later in life. The subiculum, a part of the hippocampus proper and Papez's circuit, is involved in cognitive development and is vulnerable to anesthesia-induced developmental neurodegeneration. This degeneration is manifested by acute substantial neuroapoptotic damage and permanent neuronal loss in later stages of synaptogenesis. Since synapse formation is a critical component of brain development, we examined the effects of highly neurotoxic anesthesia combination (isoflurane, nitrous oxide, and midazolam) on ultrastructural development of synapses in the rat subiculum. We found that this anesthesia, when administered at the peak of synaptogenesis, causes long-lasting injury to the subicular neuropil. This is manifested as neuropil scarcity and disarray, morphological changes indicative of mitochondria degeneration, a decrease in the number of neuronal profiles with multiple synaptic boutons and significant decreases in synapse volumetric densities. We believe that observed morphological disturbances of developing synapses may, at least in part, contribute to the learning and memory deficits that occur later in life after exposure of the immature brain to general anesthesia.
Figures





References
-
- Ahmed MG, Bedi KS, Warren MA, Kamel MM. Effects of a lengthy period of undernutrition from birth and subsequent nutritional rehabilitation on the synapse: granule cell neuron ratio in the rat dentate gyrus. J Comp Neurol. 1987;263:146–158. - PubMed
-
- Bittigau P, Sifringer M, Pohl D, Stadthaus D, Ishimaru M, Shimizu H, Ikeda M, Lang D, Speer A, Olney JW, Ikonomidou C. Apoptotic neurodegeneration following trauma is markedly enhanced in the immature brain. Ann Neurol. 1999;45:724–735. - PubMed
-
- Briones TL, Suh E, Jozsa L, Hattar H, Chai J, Wadowska M. Behaviorally-induced ultrastructural plasticity in the hippocampal region after cerebral ischemia. Brain Res. 2004;997:137–146. - PubMed
-
- Chang LW, Dudley AW, Jr, Lee YK, Katz J. Ultrastructural changes in the nervous system after chronic exposure to halothane. Exp Neurol. 1974;45:209–219. - PubMed
-
- Chang LW, Dudley AW, Jr, Katz J. Pathological changes in the nervous system following in utero exposure to halothane. Environ Res. 1976;11:40–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

