Methods for lymphatic vessel culture and gene transfection
- PMID: 19626551
- PMCID: PMC3042427
- DOI: 10.1080/10739680903120778
Methods for lymphatic vessel culture and gene transfection
Abstract
Objective: To develop the techniques needed for the specific gene/protein targeting transfection experiments in isolated lymphatic vessels, we completed two major tasks: 1) optimize the experimental conditions to maintain the viability of isolated rat lymphatic vessels in culture for sufficiently long periods of time to permit knockdown or overexpression of selected proteins/genes and 2) develop effective transfection protocols for lymphatic muscle and endothelial cells in intact lymphatic vessels without nonspecific impairment of lymphatic contractile function due to the transfection protocol itself.
Methods: Experimental protocols were developed for the maintenance of isolated lymphatic vessels under nonpressurized and pressurized conditions for 3-12 days in culture and for adenoviral gene transfection of the lymphatic muscle and endothelial cells.
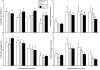
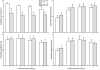
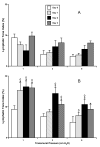
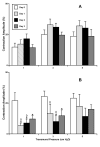
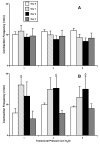
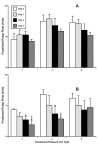
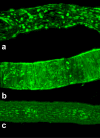
Results: The data demonstrate the effectiveness of the newly developed experimental protocols for the maintenance of isolated rat mesenteric lymphatic vessels and thoracic duct in culture up to 3-12 days without significant impairment of the parameters of their pumping and effective adenoviral/GFP transfection of lymphatic endothelial and muscle cells in isolated rat mesenteric lymphatic vessels.
Conclusions: These experimental techniques will extend the set of the modern experimental tools available to researchers investigating the physiology of lymphatic function.
Figures

Similar articles
-
Adenovirus-mediated gene transfection in the isolated lymphatic vessels.Methods Mol Biol. 2012;843:199-204. doi: 10.1007/978-1-61779-523-7_19. Methods Mol Biol. 2012. PMID: 22222534 Free PMC article.
-
Spontaneous and α-adrenoceptor-induced contractility in human collecting lymphatic vessels require chloride.Am J Physiol Heart Circ Physiol. 2018 Aug 1;315(2):H389-H401. doi: 10.1152/ajpheart.00551.2017. Epub 2018 Apr 6. Am J Physiol Heart Circ Physiol. 2018. PMID: 29631375
-
Differences in L-type Ca2+ channel activity partially underlie the regional dichotomy in pumping behavior by murine peripheral and visceral lymphatic vessels.Am J Physiol Heart Circ Physiol. 2018 May 1;314(5):H991-H1010. doi: 10.1152/ajpheart.00499.2017. Epub 2018 Jan 5. Am J Physiol Heart Circ Physiol. 2018. PMID: 29351458 Free PMC article.
-
Lymphatic biology and the microcirculation: past, present and future.Microcirculation. 2005 Jan-Feb;12(1):141-50. doi: 10.1080/10739680590900003. Microcirculation. 2005. PMID: 15804980 Review.
-
Heterogeneity in the lymphatic vascular system and its origin.Cardiovasc Res. 2016 Sep;111(4):310-21. doi: 10.1093/cvr/cvw175. Epub 2016 Jun 29. Cardiovasc Res. 2016. PMID: 27357637 Free PMC article. Review.
Cited by
-
[Influence of 7-dehydrocholesterol reductase gene silencing on the fusion of mouse palatal shelves].Hua Xi Kou Qiang Yi Xue Za Zhi. 2015 Feb;33(1):29-34. doi: 10.7518/hxkq.2015.01.007. Hua Xi Kou Qiang Yi Xue Za Zhi. 2015. PMID: 25872295 Free PMC article. Chinese.
-
In vivo visualization and quantification of collecting lymphatic vessel contractility using near-infrared imaging.Sci Rep. 2016 Mar 10;6:22930. doi: 10.1038/srep22930. Sci Rep. 2016. PMID: 26960708 Free PMC article.
-
Lymphatic lipid transport: sewer or subway?Trends Endocrinol Metab. 2010 Aug;21(8):480-7. doi: 10.1016/j.tem.2010.04.003. Epub 2010 Jun 11. Trends Endocrinol Metab. 2010. PMID: 20541951 Free PMC article. Review.
-
Engineering the Lymphatic System.Cardiovasc Eng Technol. 2011 Dec;2(4):296-308. doi: 10.1007/s13239-011-0054-6. Epub 2011 Jul 28. Cardiovasc Eng Technol. 2011. PMID: 23408477 Free PMC article.
-
Lymphatic Cannulation for Lymph Sampling and Molecular Delivery.J Immunol. 2019 Oct 15;203(8):2339-2350. doi: 10.4049/jimmunol.1900375. Epub 2019 Sep 13. J Immunol. 2019. PMID: 31519866 Free PMC article.
References
-
- Abels C, Fickweiler S, Weiderer P, Baumler W, Hofstadter F, Landthaler M, Szeimies RM. Indocyanine green (icg) and laser irradiation induce photooxidation. Arch Dermatol Res. 2000;292:404–411. - PubMed
-
- Aukland K. Arnold heller and the lymph pump. Acta Physiol Scand. 2005;185:171–180. - PubMed
-
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am. J. Physiol. 1989;257:H2059–2069. - PubMed
-
- Borisov AV. The theory of the design of the lymphangion. (in russian) Morfologiia. 1997;112:7–17. - PubMed
-
- Browse N, Burnand KG, Mortimer PS. Diseases of the lymphatics. Arnold, Hodder Headline Group; London: 2003.
Publication types
MeSH terms
Grants and funding
- R01 HL089784/HL/NHLBI NIH HHS/United States
- AG-030578/AG/NIA NIH HHS/United States
- HL-070308/HL/NHLBI NIH HHS/United States
- HL-075199/HL/NHLBI NIH HHS/United States
- R01 AG030578/AG/NIA NIH HHS/United States
- R01 HL070308/HL/NHLBI NIH HHS/United States
- K02 HL086650/HL/NHLBI NIH HHS/United States
- R01 HL096552/HL/NHLBI NIH HHS/United States
- KO2 HL-086650/HL/NHLBI NIH HHS/United States
- R01 HL080526/HL/NHLBI NIH HHS/United States
- R01 HL075199/HL/NHLBI NIH HHS/United States
- R21 HL085659/HL/NHLBI NIH HHS/United States
- HL-089784/HL/NHLBI NIH HHS/United States
- HL-080526/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

