Rlip76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer
- PMID: 19626587
- PMCID: PMC3065777
- DOI: 10.1002/ijc.24767
Rlip76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer
Abstract
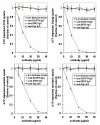
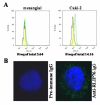
RLIP76 is a stress-responsive membrane protein implicated in the regulation of multiple cellular signaling pathways. It represents the predominant glutathione-conjugate (GS-E) transporter in cells. We have shown that RLIP76 plays a crucial role in defending cancer cells from radiation and chemotherapeutic toxin-mediated apoptosis, and that its inhibition by antibodies or depletion by siRNA or antisense causes apoptosis in a number of cancer cell types. We demonstrated for the first time that the striking anti-neoplastic effects with no evident toxicity in terms of either weight loss or metabolic effects are also demonstrable for the antibody, antisense and siRNA in a renal cell xenografts model of Caki-2 cells (Singhal et al., Cancer Res., 2009, 69: 4244). Present studies were performed to determine if RLIP76 targeting is more broadly applicable in other kidney cancer cell lines, to compare the signaling effects of RLIP76 antisense with kinase inhibitors used in treatment of renal cell carcinoma, and to determine whether kinase inhibitors were substrates for transport by RLIP76. Results of these studies show that sorafenib as well as sunitinib are substrates for transport by RLIP76 thus are competitive inhibitors of GS-E transport. Furthermore, kinase inhibition in the ERK as well as PI3K pathways by RLIP76 depletion is more profound and consistent and is more widely apparent in a number of renal carcinoma cell lines. These studies offer strong support for our overall hypothesis that RLIP76 is an overarching anti-apoptosis mechanism that, if inhibited, can be more broadly effective in the treatment of renal cell carcinoma.
Figures






References
-
- Yao M, Yoshida M, Kishida T, Nakaigawa N, Baba M, Kobayashi K, Miura T, Moriyama M, Nagashima Y, Nakatani Y, Kubota Y, Kondo K. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94:1569–75. - PubMed
-
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–7. - PubMed
-
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. - PubMed
-
- Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–7. - PubMed
-
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

