Fibulin-4 regulates expression of the tropoelastin gene and consequent elastic-fibre formation by human fibroblasts
- PMID: 19627254
- PMCID: PMC3024593
- DOI: 10.1042/BJ20090993
Fibulin-4 regulates expression of the tropoelastin gene and consequent elastic-fibre formation by human fibroblasts
Abstract
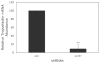
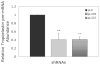
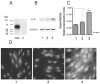
Elastic fibres are essential for normal physiology in numerous tissues, including arteries, lungs and skin. Fibulin-4 is an elastic-fibre-associated glycoprotein that is indispensable for elastic-fibre formation in mice. However, the mechanism by which fibulin-4 executes this function remains to be determined. Here, we established an in vitro functional assay system in which fibulin-4 was knocked down in human foreskin fibroblasts using siRNA (small interfering RNA) technology. With two different siRNAs, substantial knockdown of fibulin-4 was achieved, and this suppression was associated with impaired elastic-fibre formation by the fibroblasts. Real-time reverse transcription-PCR analysis showed that knockdown of fibulin-4 expression was accompanied by reduced expression of tropoelastin mRNA. Further analysis showed that this decrease was caused by transcriptional down-regulation of tropoelastin. This effect was selective, since the mRNA level of other elastic-fibre-associated proteins, including fibrillin-1, lysyl oxidase and lysyl oxidase-like-1, was not affected. Moreover, addition of conditioned medium from cultures of CHO (Chinese-hamster ovary) cells overexpressing fibulin-4 stimulated tropoelastin expression and elastic-fibre formation in cultures of Williams-Beuren-syndrome fibroblasts. Knocking down or knocking out fibulin-4 in mice led to a decrease in tropoelastin expression in the aorta. These results indicate that fibulin-4, considered as a structural protein, may also participate in regulating elastic-fibre formation in human cells through an unanticipated mechanism, namely the regulation of tropoelastin expression.
Figures







Similar articles
-
PKCε Increases Extracellular Elastin and Fibulin-5/DANCE in Dermal Fibroblasts.Cell Physiol Biochem. 2018;46(1):291-302. doi: 10.1159/000488430. Epub 2018 Mar 23. Cell Physiol Biochem. 2018. PMID: 29590645
-
Differential regulation of elastic fiber formation by fibulin-4 and -5.J Biol Chem. 2009 Sep 4;284(36):24553-67. doi: 10.1074/jbc.M109.019364. Epub 2009 Jul 1. J Biol Chem. 2009. PMID: 19570982 Free PMC article.
-
Fibulin-5 accelerates elastic fibre assembly in human skin fibroblasts.Exp Dermatol. 2008 Oct;17(10):837-42. doi: 10.1111/j.1600-0625.2008.00709.x. Epub 2008 Mar 13. Exp Dermatol. 2008. PMID: 18341572
-
Elastic Fibre Proteins in Elastogenesis and Wound Healing.Int J Mol Sci. 2022 Apr 7;23(8):4087. doi: 10.3390/ijms23084087. Int J Mol Sci. 2022. PMID: 35456902 Free PMC article. Review.
-
Elastic tissue disruption is a major pathogenic factor to human vascular disease.Mol Biol Rep. 2021 May;48(5):4865-4878. doi: 10.1007/s11033-021-06478-8. Epub 2021 Jun 15. Mol Biol Rep. 2021. PMID: 34129188 Review.
Cited by
-
Elastogenic protein expression of a highly elastic murine spinal ligament: the ligamentum flavum.PLoS One. 2012;7(6):e38475. doi: 10.1371/journal.pone.0038475. Epub 2012 Jun 7. PLoS One. 2012. PMID: 22685574 Free PMC article.
-
Fibulin-4 deficiency increases TGF-β signalling in aortic smooth muscle cells due to elevated TGF-β2 levels.Sci Rep. 2015 Nov 26;5:16872. doi: 10.1038/srep16872. Sci Rep. 2015. PMID: 26607280 Free PMC article.
-
Decreased mitochondrial respiration in aneurysmal aortas of Fibulin-4 mutant mice is linked to PGC1A regulation.Cardiovasc Res. 2018 Nov 1;114(13):1776-1793. doi: 10.1093/cvr/cvy150. Cardiovasc Res. 2018. PMID: 29931197 Free PMC article.
-
Elastin in the Liver.Front Physiol. 2016 Oct 25;7:491. doi: 10.3389/fphys.2016.00491. eCollection 2016. Front Physiol. 2016. PMID: 27826254 Free PMC article. Review.
-
Role of Fibulins in Embryonic Stage Development and Their Involvement in Various Diseases.Biomolecules. 2021 May 2;11(5):685. doi: 10.3390/biom11050685. Biomolecules. 2021. PMID: 34063320 Free PMC article. Review.
References
-
- Mecham R, Davis E. Elastic fibre structure and assembly. In: Yurchenco P, Birk D, Mecham R, editors. Extracellular Matrix Assembly and Structure. Academic Press; San Diego: 1994. pp. 281–314.
-
- Vrhovski B, Weiss AS. Biochemistry of tropoelastin. Eur J Biochem. 1998;258:1–18. - PubMed
-
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. - PubMed
-
- Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fibre assembly. J Biol Chem. 2006;281:3698–3710. - PubMed
-
- Hinek A. Nature and the multiple functions of the 67-kD elastin-/laminin binding protein. Cell Adhes Commun. 1994;2:185–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

