The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae
- PMID: 19627615
- PMCID: PMC2726147
- DOI: 10.1186/1471-230X-9-58
The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae
Abstract
Background: GP2 is the major membrane protein present in the pancreatic zymogen granule, and is cleaved and released into the pancreatic duct along with exocrine secretions. The function of GP2 is unknown. GP2's amino acid sequence is most similar to that of uromodulin, which is secreted by the kidney. Recent studies have demonstrated uromodulin binding to bacterial Type 1 fimbria. The fimbriae serve as adhesins to host receptors. The present study examines whether GP2 also shares similar binding properties to bacteria with Type 1 fimbria. Commensal and pathogenic bacteria, including E. coli and Salmonella, express type 1 fimbria.
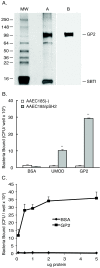
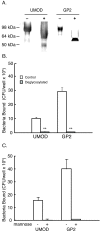
Methods: An in vitro binding assay was used to assay the binding of recombinant GP2 to defined strains of E. coli that differ in their expression of Type 1 fimbria or its subunit protein, FimH. Studies were also performed to determine whether GP2 binding is dependent on the presence of mannose residues, which is a known determinant for FimH binding.
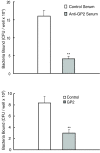
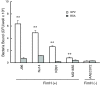
Results: GP2 binds E. coli that express Type 1 fimbria. Binding is dependent on GP2 glycosylation, and specifically the presence of mannose residues.
Conclusion: GP2 binds to Type 1 fimbria, a bacterial adhesin that is commonly expressed by members of the Enterobacteriacae family.
Figures
References
-
- Hoops TC, Rindler MJ. Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. J BiolChem. 1991;266:4257–4263. - PubMed
-
- Dittie A, Kern H-F. The major zymogen granule membrane protein GP-2 in the rat pancreas is not involved in granule formation. Eur J Cell Biol. 1992;58:243–258. - PubMed
-
- Lowe AW, Luthen RE, Wong SME, Grendell JH. The zymogen granule protein, GP-2, is elevated in a rat model for acute pancreatitis. Gastroenterology. 1994;107:1819–1827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

