Dynamics and stability in maturation of a T=4 virus
- PMID: 19627990
- PMCID: PMC2766526
- DOI: 10.1016/j.jmb.2009.07.038
Dynamics and stability in maturation of a T=4 virus
Abstract
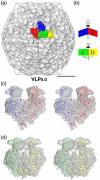
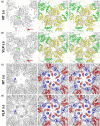
Nudaurelia capensis omega virus is a T=4, icosahedral virus with a bipartite, positive-sense RNA genome. Expression of the coat protein gene in a baculovirus system was previously shown to result in the formation of procapsids when purified at pH 7.6. Procapsids are round, porous particles (480 A diameter) and have T=4 quasi-symmetry. Reduction of pH from 7.6 to 5.0 resulted in virus-like particles (VLP(5.0)) that are morphologically identical with authentic virions, with an icosahedral-shaped capsid and a maximum dimension of 410 A. VLP(5.0) undergoes a maturation cleavage between residues N570 and F571, creating the covalently independent gamma peptide (residues 571-641) that remains associated with the particle. This cleavage also occurs in authentic virions, and in each case, it renders the morphological change irreversible (i.e., capsids do not expand when the pH is raised back to 7.6). However, a non-cleavable mutant, N570T, undergoes the transition reversibly (NT(7.6)<-->NT(5.0)). We used electron cryo-microscopy and three-dimensional image reconstruction to study the icosahedral structures of NT(7.6), NT(5.0), and VLP(5.0) at about 8, 6, and 6 A resolution, respectively. We employed the 2. 8-A X-ray model of the mature virus, determined at pH 7.0 (XR(7.0)), to establish (1) how and why procapsid and capsid structures differ, (2) why lowering pH drives the transition, and (3) why the non-cleaving NT(5.0) is reversible. We show that procapsid assembly minimizes the differences in quaternary interactions in the particle. The two classes of 2-fold contacts in the T=4 surface lattice are virtually identical, both mediated by similarly positioned but dynamic gamma peptides. Furthermore, quasi and icosahedral 3-fold interactions are indistinguishable. Maturation results from neutralizing the repulsive negative charge at subunit interfaces with significant differentiation of quaternary interactions (one 2-fold becomes flat, mediated by a gamma peptide, while the other is bent with the gamma peptide disordered) and dramatic stabilization of the particle. The gamma peptide at the flat contact remains dynamic when cleavage cannot occur (NT(5.0)) but becomes totally immobilized by noncovalent interactions after cleavage (VLP(5.0)).
Figures




References
-
- Hanzlik TN, Gordon KH. The Tetraviridae. Adv Virus Res. 1997;48:101–68. - PubMed
-
- Agrawal DK, Johnson JE. Assembly of the T = 4 Nudaurelia capensis omega virus capsid protein, post-translational cleavage, and specific encapsidation of its mRNA in a baculovirus expression system. Virology. 1995;207:89–97. - PubMed
-
- Canady MA, Tihova M, Hanzlik TN, Johnson JE, Yeager M. Large conformational changes in the maturation of a simple RNA virus, nudaurelia capensis omega virus (NomegaV) J Mol Biol. 2000;299:573–84. - PubMed
-
- Canady MA, Tsuruta H, Johnson JE. Analysis of rapid, large-scale protein quaternary structural changes: time-resolved X-ray solution scattering of Nudaurelia capensis omega virus (NomegaV) maturation. J Mol Biol. 2001;311:803–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

