NOX and inflammation in the vascular adventitia
- PMID: 19628034
- PMCID: PMC3061339
- DOI: 10.1016/j.freeradbiomed.2009.07.022
NOX and inflammation in the vascular adventitia
Abstract
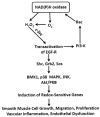
Vascular inflammation has traditionally been thought to be initiated at the luminal surface and progress through the media toward the adventitial layer. In recent years, however, evidence has emerged suggesting that the vascular adventitia is activated early in a variety of cardiovascular diseases and that it plays an important role in the initiation and progression of vascular inflammation. Adventitial fibroblasts have been shown to produce substantial amounts of NAD(P)H oxidase-derived reactive oxygen species (ROS) in response to vascular injury. Additionally, inflammatory cytokines, lipids, and various hormones, implicated in fibroblast proliferation and migration, lead to recruitment of inflammatory cells to the adventitial layer and impairment of endothelium-dependent relaxation. Early in the development of vascular disease, there is clear evidence for progression toward a denser vasa vasorum which delivers oxygen and nutrients to an increasingly hypoxic and nutrient-deficient media. This expanded vascularization appears to provide enhanced delivery of inflammatory cells to the adventitia and outer media. Combined adventitial fibroblast and inflammatory cell-derived ROS therefore are expected to synergize their local effect on adventitial parenchymal cells, leading to further cytokine release and a feed-forward propagation of adventitial ROS production. In fact, data from our laboratory and others suggest a broader paracrine positive feedback role for adventitia-derived ROS in medial smooth muscle cell hypertrophy and neointimal hyperplasia. A likely candidate responsible for the adventitia-derived paracrine signaling across the vessel wall is the superoxide anion metabolite hydrogen peroxide, which is highly stable, cell permeant, and capable of activating downstream signaling mechanisms in smooth muscle cells, leading to phenotypic modulation of smooth muscle cells. This review addresses the role of adventitial NAD(P)H oxidase-derived ROS from a nontraditional, perivascular vantage of promoting vascular inflammation and will discuss how ROS derived from adventitial NAD(P)H oxidases may be a catalyst for vascular remodeling and dysfunction.
Figures

References
-
- Campbell SC, Moffatt RJ, Stamford BA. Smoking and smoking cessation-The relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis. 2008 - PubMed
-
- Ishigaki Y, Katagiri H, Gao J, Yamada T, Imai J, Uno K, Hasegawa Y, Kaneko K, Ogihara T, Ishihara H, Sato Y, Takikawa K, Nishimichi N, Matsuda H, Sawamura T, Oka Y. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118:75–83. - PubMed
-
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. The aging endothelium, cardiovascular risk and disease. Exp Physiol. 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

