Nox proteins in signal transduction
- PMID: 19628035
- PMCID: PMC2763943
- DOI: 10.1016/j.freeradbiomed.2009.07.023
Nox proteins in signal transduction
Abstract
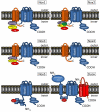
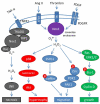
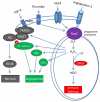
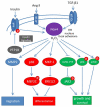
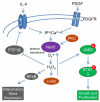
The NADPH oxidase (Nox) family of superoxide (O(2)(*-)) and hydrogen peroxide (H(2)O(2))-producing proteins has emerged as an important source of reactive oxygen species (ROS) in signal transduction. ROS produced by Nox proteins Nox1-5 and Duox1/2 are now recognized to play essential roles in the physiology of the brain, the immune system, the vasculature, and the digestive tract as well as in hormone synthesis. Nox-derived ROS have been implicated in regulation of cytoskeletal remodeling, gene expression, proliferation, differentiation, migration, and cell death. These processes are tightly controlled and reversible. In this review, we will discuss recent literature on Nox protein tissue distribution, subcellular localization, activation, and the resulting signal transduction mechanisms.
Figures
References
-
- Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007;89:1107–1112. - PubMed
-
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. - PubMed
-
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

