Donor graft interferon regulatory factor-1 gene transfer worsens liver transplant ischemia/reperfusion injury
- PMID: 19628072
- PMCID: PMC2739669
- DOI: 10.1016/j.surg.2009.06.011
Donor graft interferon regulatory factor-1 gene transfer worsens liver transplant ischemia/reperfusion injury
Abstract
Background: Liver ischemia and reperfusion (IR) injury is a phenomenon that leads to graft dysfunction after liver transplantation. Understanding the molecular mechanisms behind this process is crucial to developing strategies to prevent short- and long-term graft dysfunction. The purpose of this study was to explore the role of the transcription factor interferon regulatory factor-1 (IRF-1) in a model of orthotopic rat liver transplantation.
Methods: Orthotopic syngeneic LEW rat liver transplantation (OLT) was performed after 18 or 3 hours preservation in cold University of Wisconsin solution. Adenovirus-expressing IRF-1 (AdIRF-1) or control gene vector (Adnull) was delivered to the liver by donor intravenous pretreatment 4 days before graft harvesting. Uninfected grafts also served as controls. Recipients were humanely killed 1-24 hours post-transplantation.
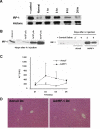
Results: Rats that underwent OLT with long-term preserved grafts (18 hours) displayed increased hepatic nuclear expression of IRF-1 protein at 1 and 3 hours. Rats pretreated with AdIRF-1 before transplantation had elevated alanine aminotransferase levels and increased expression of interferon (IFN)-beta, IFN-gamma, interleukin-12, and inducible nitric oxide synthase in the short-term period (3 hours) when compared with donor livers pretreated with Adnull. AdIRF-1 pretreated donor livers also exhibited increased susceptibility to early apoptosis in the transplanted grafts as shown by increased terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) staining and expression of cleaved caspase-3. Additionally, AdIRF-1 pretreated donor livers had increased activation of the MAP kinase Jun N-terminal kinase as compared with Adnull pretreated donor livers.
Conclusion: IRF-1 is an important regulator of IR injury after OLT in rats. Targeting of IRF-1 may be a potential strategy to ameliorate ischemic liver injury after transplantation to minimize organ dysfunction.
Figures




Similar articles
-
Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G666-73. doi: 10.1152/ajpgi.00050.2012. Epub 2012 Jun 28. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22744333 Free PMC article.
-
Donor graft adenoviral iNOS gene transfer ameliorates rat liver transplant preservation injury and improves survival.Hepatology. 2006 Mar;43(3):464-73. doi: 10.1002/hep.21067. Hepatology. 2006. PMID: 16496305
-
Native macrophages genetically modified to express heme oxygenase 1 protect rat liver transplants from ischemia/reperfusion injury.Liver Transpl. 2011 Feb;17(2):201-10. doi: 10.1002/lt.22214. Liver Transpl. 2011. PMID: 21280193 Free PMC article.
-
Carbon monoxide induces hypothermia tolerance in Kupffer cells and attenuates liver ischemia/reperfusion injury in rats.Liver Transpl. 2011 Dec;17(12):1457-66. doi: 10.1002/lt.22415. Liver Transpl. 2011. PMID: 21850691 Free PMC article.
-
CD4+ T Cell NRF2 Signaling Improves Liver Transplantation Outcomes by Modulating T Cell Activation and Differentiation.Antioxid Redox Signal. 2023 Mar;38(7-9):670-683. doi: 10.1089/ars.2022.0094. Epub 2023 Mar 1. Antioxid Redox Signal. 2023. PMID: 36070449 Free PMC article. Review.
Cited by
-
Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G666-73. doi: 10.1152/ajpgi.00050.2012. Epub 2012 Jun 28. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22744333 Free PMC article.
-
Preconditioning with physiological levels of ethanol protect kidney against ischemia/reperfusion injury by modulating oxidative stress.PLoS One. 2011;6(10):e25811. doi: 10.1371/journal.pone.0025811. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022451 Free PMC article.
-
Interferon regulatory factor 1 priming of tumour-derived exosomes enhances the antitumour immune response.Br J Cancer. 2018 Jan;118(1):62-71. doi: 10.1038/bjc.2017.389. Epub 2017 Nov 7. Br J Cancer. 2018. PMID: 29112686 Free PMC article.
-
The Role of Interferon Regulatory Factors in Liver Diseases.Int J Mol Sci. 2024 Jun 22;25(13):6874. doi: 10.3390/ijms25136874. Int J Mol Sci. 2024. PMID: 38999981 Free PMC article. Review.
-
IRF-1 promotes liver transplant ischemia/reperfusion injury via hepatocyte IL-15/IL-15Rα production.J Immunol. 2015 Jun 15;194(12):6045-56. doi: 10.4049/jimmunol.1402505. Epub 2015 May 11. J Immunol. 2015. PMID: 25964490 Free PMC article.
References
-
- Adam R, Bismuth H, Diamond T, Ducot B, Morino M, Astarcioglu I, et al. Effect of extended cold ischaemia with UW solution on graft function after liver transplantation. Lancet. 1992;340:1373–1376. - PubMed
-
- Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. - PubMed
-
- Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am. J. Transplant. 2006;6:783–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

