Design, synthesis and biological evaluation of novel pyridine derivatives as anticancer agents and phosphodiesterase 3 inhibitors
- PMID: 19628397
- PMCID: PMC4980832
- DOI: 10.1016/j.bmc.2009.06.063
Design, synthesis and biological evaluation of novel pyridine derivatives as anticancer agents and phosphodiesterase 3 inhibitors
Abstract
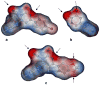
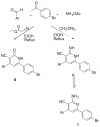
Two series of 4,6-diaryl-2-imino-1,2-dihydropyridine-3-carbonitriles and their isosteric 4,6-diaryl-2-oxo-1,2-dihydropyridine-3-carbonitriles were synthesized through a combinatorial approach. The prepared analogues were evaluated for their in vitro capacity to inhibit PDE3A and the growth of the human HT-29 colon adenocarcinoma tumor cell line. Compound 6-(4-bromophenyl)-4-(2-ethoxyphenyl)-2-imino-1,2-dihydropyridine-3-carbonitrile (Id) exhibited the strongest PDE3 inhibition when cGMP but not cAMP is the substrate with a IC(50)of 27microM, which indicates a highly selective mechanism of enzyme inhibition. On the other hand, compound 6-(1,3-benzodioxol-5-yl)-4-(2-ethoxyphenyl)-2-imino-1,2-dihydropyridine-3-carbonitrile (Ii) was the most active in inhibiting colon tumor cell growth with a IC(50) of 3microM. The electronic effects, steric effects and conformational aspects of Id seem to be the most crucial for the PDE3 inhibition. Meanwhile, steric factors and the H-bonding capability seem to be the most important factors for tumor cell growth inhibitory activity. Conversely, there is no direct correlation between PDE3 inhibition and anticancer activity for the prepared compounds. An in silico docking experiment indicates the potential involvement of other potential molecular targets such as PIM-1 kinase to explain its tumor cell growth inhibitory activity.
Figures

References
-
- Cheney IW, Yan S, Appleby T, Walker H, Vo T, Yao N, Hamatake R, Hong Z, Wu JZ. Bioorg Med Chem Lett. 2007;17:1679. - PubMed
-
- Wendt MD, Sun C, Kunzer A, Sauer D, Sarris K, Hoff E, Yu L, Nettesheim DG, Chen J, Jin S, Comess KM, Fan Y, Anderson SN, Isaac B. Bioorg Med Chem Lett. 2007;17:3122. - PubMed
-
- Aqui NA, Vonderheide RH. Cancer Biol Ther. 2008;7:1888. - PubMed
-
- Ambrosini G, Adida C, Altieri DC. Nat Med. 1997;3:917. - PubMed
-
- Gary P, Soh JW, Mao Y, Kim MG, Pamukcu R, Li H, Thompson WJ, Weinstein IB. Clin Cancer Res. 2000;6:4136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

