A randomized placebo-controlled study of tamoxifen after adjuvant chemotherapy in premenopausal women with early breast cancer (National Cancer Institute of Canada--Clinical Trials Group Trial, MA.12)
- PMID: 19628570
- PMCID: PMC2813306
- DOI: 10.1093/annonc/mdp326
A randomized placebo-controlled study of tamoxifen after adjuvant chemotherapy in premenopausal women with early breast cancer (National Cancer Institute of Canada--Clinical Trials Group Trial, MA.12)
Abstract
Background: In the early 1990s, the role of adjuvant tamoxifen in premenopausal women with early breast cancer (EBC) was not established. Similarly, optimum timing relative to adjuvant chemotherapy and efficacy of tamoxifen in hormone receptor-negative tumors were unclear.
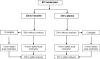
Patients and methods: Premenopausal women with EBC, any hormone receptor status, after surgery received standard adjuvant chemotherapy [doxorubicin (adriamycin)/cyclophosphamide, cyclophosphamide/methotrexate/5-fluorouracil, or cyclophosphamide/epirubicin/5-fluorouracil] followed by randomization to tamoxifen or placebo for 5 years. Outcomes were overall survival (OS), disease-free survival (DFS), toxicity, and compliance with therapy.
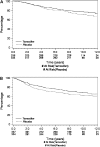
Results: Median follow-up for 672 women was 9.7 years. Multivariate analysis showed improved DFS [78.2% versus 71.3% at 5 years; hazard ratio (HR) 0.77; P = 0.056] and a trend for improved OS (86.6% versus 82.1% at 5 years; HR 0.78; P = 0.12). There was no evidence of greater benefit for the receptor-positive subgroup. Compliance with treatment was suboptimal in both arms, with 103 (31%) women on tamoxifen and 70 (21%) on placebo-stopping therapy early because of toxicity, refusal, or other choices.
Conclusions: Adjuvant tamoxifen, given after chemotherapy to premenopausal women with EBC, improved 5-year DFS. Poor compliance may have reduced treatment efficacy.
Figures
Similar articles
-
Addition of adjuvant tamoxifen to cyclophosphamide, methotrexate and 5-fluorouracil for premenopausal women with oestrogen receptor-positive breast cancer.Asian J Surg. 2003 Jul;26(3):163-8. doi: 10.1016/S1015-9584(09)60377-8. Asian J Surg. 2003. PMID: 12925292
-
Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93.J Clin Oncol. 2006 Mar 20;24(9):1332-41. doi: 10.1200/JCO.2005.03.0783. Epub 2006 Feb 27. J Clin Oncol. 2006. PMID: 16505417 Clinical Trial.
-
Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group.J Natl Cancer Inst. 1996 Dec 18;88(24):1828-33. doi: 10.1093/jnci/88.24.1828. J Natl Cancer Inst. 1996. PMID: 8961972 Clinical Trial.
-
Reducing the risk for breast cancer recurrence after completion of tamoxifen treatment in postmenopausal women.Clin Ther. 2007 Aug;29(8):1535-47. doi: 10.1016/j.clinthera.2007.08.013. Clin Ther. 2007. PMID: 17919537 Review.
-
Optimal systemic therapy for premenopausal women with hormone receptor-positive breast cancer.Breast. 2013 Aug;22 Suppl 2:S165-70. doi: 10.1016/j.breast.2013.07.032. Breast. 2013. PMID: 24074781 Review.
Cited by
-
Estrogenic Activity and Risk of Invasive Breast Cancer Among Postmenopausal Women in the Nurses' Health Study.Cancer Epidemiol Biomarkers Prev. 2022 Apr 1;31(4):831-838. doi: 10.1158/1055-9965.EPI-21-1157. Cancer Epidemiol Biomarkers Prev. 2022. PMID: 35131884 Free PMC article.
-
Molecular characterization of basal-like and non-basal-like triple-negative breast cancer.Oncologist. 2013;18(2):123-33. doi: 10.1634/theoncologist.2012-0397. Epub 2013 Feb 12. Oncologist. 2013. PMID: 23404817 Free PMC article. Review.
-
Oral Endocrine Therapy Nonadherence, Adverse Effects, Decisional Support, and Decisional Needs in Women With Breast Cancer.Cancer Nurs. 2018 Jan/Feb;41(1):E9-E18. doi: 10.1097/NCC.0000000000000430. Cancer Nurs. 2018. PMID: 27532743 Free PMC article. Review.
-
Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials.Lancet. 2011 Aug 27;378(9793):771-84. doi: 10.1016/S0140-6736(11)60993-8. Epub 2011 Jul 28. Lancet. 2011. PMID: 21802721 Free PMC article.
-
Standard of care and promising new agents for triple negative metastatic breast cancer.Cancers (Basel). 2014 Oct 24;6(4):2187-223. doi: 10.3390/cancers6042187. Cancers (Basel). 2014. PMID: 25347122 Free PMC article. Review.
References
-
- Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31 000 recurrences and 24 000 deaths among 75 000 women. Lancet. 1992;339:71–85. - PubMed
-
- Albain KS, Green SJ, Ravdin PM, et al. Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: initial results from intergroup trial 0100 (SWOG-8814) Proc Am Soc Clin Oncol. 2002;21:37a. (Abstr 143)
-
- Osborne CK. Effects of estrogens and antiestrogens on cell proliferation: implications for the treatment of breast cancer. Cancer Treat Res. 1988;39:111–129. - PubMed
-
- Goldenberg GJ, Froese EK. Antagonism of the cytocidal activity and uptake of melphalan by tamoxifen in human breast cancer cells in vitro. Biochem Pharmacol. 1985;34:763–770. - PubMed
-
- Walshe JM, Neelima D, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical