Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status
- PMID: 19628573
- PMCID: PMC2754689
- DOI: 10.1210/en.2009-0642
Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status
Abstract
The medial basal hypothalamus, including the arcuate nucleus (ARC) and the ventromedial hypothalamic nucleus (VMH), integrates signals of energy status to modulate metabolism and energy balance. Leptin and feeding regulate the mammalian target of rapamycin complex 1 (mTORC1) in the hypothalamus, and hypothalamic mTORC1 contributes to the control of feeding and energy balance. To determine the mechanisms by which leptin modulates mTORC1 in specific hypothalamic neurons, we immunohistochemically assessed the mTORC1-dependent phosphorylation of ribosomal protein S6 (pS6). In addition to confirming the modulation of ARC mTORC1 activity by acute leptin treatment, this analysis revealed the robust activation of mTORC1-dependent ARC pS6 in response to fasting and leptin deficiency in leptin receptor-expressing Agouti-related protein neurons. In contrast, fasting and leptin deficiency suppress VMH mTORC1 signaling. The appropriate regulation of ARC mTORC1 by mutant leptin receptor isoforms correlated with their ability to suppress the activity of Agouti-related protein neurons, suggesting the potential stimulation of mTORC1 by the neuronal activity. Indeed, fasting- and leptin deficiency-induced pS6-immunoreactivity (IR) extensively colocalized with c-Fos-IR in ARC and VMH neurons. Furthermore, ghrelin, which activates orexigenic ARC neurons, increased ARC mTORC1 activity and induced colocalized pS6- and c-Fos-IR. Thus, neuronal activity promotes mTORC1/pS6 in response to signals of energy deficit. In contrast, insulin, which activates mTORC1 via the phosphatidylinositol 3-kinase pathway, increased ARC and VMH pS6-IR in the absence of neuronal activation. The regulation of mTORC1 in the basomedial hypothalamus thus varies by cell and stimulus type, as opposed to responding in a uniform manner to nutritional and hormonal perturbations.
Figures



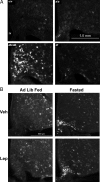
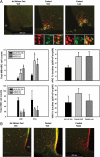
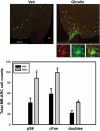
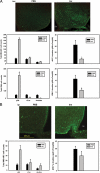
References
-
- Tierney EF, Gregg EW, Narayan KM 2006 Leading causes of death in the United States. JAMA 295:383 - PubMed
-
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF 2003 Lifetime risk for diabetes mellitus in the United States. JAMA 290:1884–1890 - PubMed
-
- Ogden CL, Carroll MD, Flegal KM 2003 Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin North Am 32:741–760, vii - PubMed
-
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 - PubMed
-
- Friedman JM, Halaas JL 1998 Leptin and the regulation of body weight in mammals. Nature 395:763–770 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

