A preformed signaling complex mediates GnRH-activated ERK phosphorylation of paxillin and FAK at focal adhesions in L beta T2 gonadotrope cells
- PMID: 19628583
- PMCID: PMC5419161
- DOI: 10.1210/me.2008-0260
A preformed signaling complex mediates GnRH-activated ERK phosphorylation of paxillin and FAK at focal adhesions in L beta T2 gonadotrope cells
Abstract
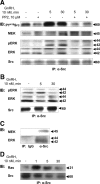
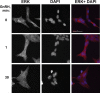
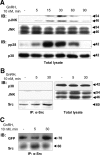
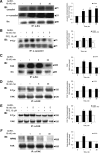
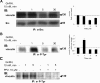
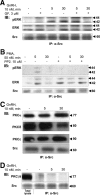
Most receptor tyrosine kinases and G protein-coupled receptors (GPCRs) operate via a limited number of MAPK cascades but still exert diverse functions, and therefore signal specificity remains an enigma. Also, most GPCR ligands utilize families of receptors for mediation of diverse biological actions; however, the mammalian type I GnRH receptor (GnRHR) seems to be the sole receptor mediating GnRH-induced gonadotropin synthesis and release. Signaling complexes associated with GPCRs may thus provide the means for signal specificity. Here we describe a signaling complex associated with the GnRHR, which is a unique GPCR lacking a C-terminal tail. Unlike other GPCRs, this signaling complex is preformed, and exposure of L beta T2 gonadotropes to GnRH induces its dynamic rearrangement. The signaling complex includes c-Src, protein kinase C delta, -epsilon, and -alpha, Ras, MAPK kinase 1/2, ERK1/2, tubulin, focal adhesion kinase (FAK), paxillin, vinculin, caveolin-1, kinase suppressor of Ras-1, and the GnRHR. Exposure to GnRH (5 min) causes MAPK kinase 1/2, ERK1/2, tubulin, vinculin, and the GnRHR to detach from c-Src, but they reassociate within 30 min. On the other hand, FAK, paxillin, the protein kinase Cs, and caveolin-1 stay bound to c-Src, whereas kinase suppressor of Ras-1 appears in the complex only 30 min after GnRH stimulation. GnRH was found to activate ERK1/2 in the complex in a c-Src-dependent manner, and the activated ERK1/2 subsequently phosphorylates FAK and paxillin. In parallel, caveolin-1, FAK, vinculin, and paxillin are phosphorylated on Tyr residues apparently by GnRH-activated c-Src. Receptor tyrosine kinases and GPCRs translocate ERK1/2 to the nucleus to phosphorylate and activate transcription factors. We therefore propose that the role of the multiprotein signaling complex is to sequester a cytosolic pool of activated ERK1/2 to phosphorylate FAK and paxillin at focal adhesions.
Figures



Similar articles
-
Cytoskeletal reorganization dependence of signaling by the gonadotropin-releasing hormone receptor.J Biol Chem. 2004 Jan 16;279(3):1980-93. doi: 10.1074/jbc.M309827200. Epub 2003 Oct 14. J Biol Chem. 2004. PMID: 14559894
-
Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness.Mol Biol Cell. 2005 Sep;16(9):4316-28. doi: 10.1091/mbc.e05-02-0131. Epub 2005 Jul 6. Mol Biol Cell. 2005. PMID: 16000375 Free PMC article.
-
Transforming growth factor-beta1 effects on endothelial monolayer permeability involve focal adhesion kinase/Src.Am J Respir Cell Mol Biol. 2007 Oct;37(4):485-93. doi: 10.1165/rcmb.2006-0439OC. Epub 2007 Jun 21. Am J Respir Cell Mol Biol. 2007. PMID: 17585111 Free PMC article.
-
Paxillin interactions.J Cell Sci. 2000 Dec;113 Pt 23:4139-40. doi: 10.1242/jcs.113.23.4139. J Cell Sci. 2000. PMID: 11069756 Review.
-
Intracellular signaling pathways mediated by the gonadotropin-releasing hormone (GnRH) receptor.Arch Med Res. 2001 Nov-Dec;32(6):499-509. doi: 10.1016/s0188-4409(01)00331-9. Arch Med Res. 2001. PMID: 11750725 Review.
Cited by
-
FOXO1 is regulated by insulin and IGF1 in pituitary gonadotropes.Mol Cell Endocrinol. 2015 Apr 15;405:14-24. doi: 10.1016/j.mce.2015.02.006. Epub 2015 Feb 9. Mol Cell Endocrinol. 2015. PMID: 25676570 Free PMC article.
-
Using automated imaging to interrogate gonadotrophin-releasing hormone receptor trafficking and function.Mol Cell Endocrinol. 2011 Jan 15;331(2):194-204. doi: 10.1016/j.mce.2010.07.008. Epub 2010 Aug 3. Mol Cell Endocrinol. 2011. PMID: 20688134 Free PMC article. Review.
-
Gq protein-induced apoptosis is mediated by AKT kinase inhibition that leads to protein kinase C-induced c-Jun N-terminal kinase activation.J Biol Chem. 2011 Sep 2;286(35):31022-31031a. doi: 10.1074/jbc.M111.247726. Epub 2011 Jul 13. J Biol Chem. 2011. Retraction in: J Biol Chem. 2017 May 26;292(21):8848. doi: 10.1074/jbc.A111.247726. PMID: 21757743 Free PMC article. Retracted.
-
Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes.Endocrinology. 2010 Oct;151(10):4882-93. doi: 10.1210/en.2009-1483. Epub 2010 Aug 4. Endocrinology. 2010. PMID: 20685880 Free PMC article.
-
Differential signaling of the GnRH receptor in pituitary gonadotrope cell lines and prostate cancer cell lines.Mol Cell Endocrinol. 2013 Apr 30;369(1-2):107-18. doi: 10.1016/j.mce.2013.01.010. Epub 2013 Feb 1. Mol Cell Endocrinol. 2013. PMID: 23380421 Free PMC article.
References
-
- Naor Z1990. Signal transduction mechanisms of Ca2+ mobilizing hormones: the case of gonadotropin-releasing hormone. Endocr Rev 11:326–353 - PubMed
-
- Sealfon SC, Weinstein H, Millar RP1997. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev 18:180–205 - PubMed
-
- Shacham S, Harris D, Ben-Shlomo H, Cohen I, Bonfil D, Przedecki F, Lewy H, Ashkenazi IE, Seger R, Naor Z2001. Mechanism of GnRH receptor signaling on gonadotropin release and gene expression in pituitary gonadotrophs. Vitam Horm 63:63–90 - PubMed
-
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR2004. Gonadotropin-releasing hormone receptors. Endocr Rev 25:235–275 - PubMed
-
- Dobkin-Bekman M, Naidich M, Pawson AJ, Millar RP, Seger R, Naor Z2006. Activation of Mitogen-activated protein kinase (MAPK) by GnRH is cell-context dependent. Mol Cell Endocrinol 252:184–190 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

