Enteropathogenic Escherichia coli inhibits intestinal vitamin B1 (thiamin) uptake: studies with human-derived intestinal epithelial Caco-2 cells
- PMID: 19628653
- PMCID: PMC2763801
- DOI: 10.1152/ajpgi.00250.2009
Enteropathogenic Escherichia coli inhibits intestinal vitamin B1 (thiamin) uptake: studies with human-derived intestinal epithelial Caco-2 cells
Abstract
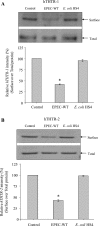
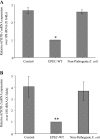
Infection with the gram-negative enteropathogenic Escherichia coli (EPEC), a food-borne pathogen, represents a significant risk to human health. Whereas diarrhea is a major consequence of this infection, malnutrition also occurs especially in severe and prolonged cases, which may aggravate the health status of the infected hosts. Here we examined the effect of EPEC infection on the intestinal uptake of the water-soluble vitamin B1 (thiamin) using an established human intestinal epithelial Caco-2 cell model. The results showed that infecting Caco-2 cells with wild-type EPEC (but not with nonpathogenic E. coli, killed EPEC, or filtered supernatant) leads to a significant (P < 0.01) inhibition in thiamin uptake. Kinetic parameters of both the nanomolar (mediated by THTR-2) and the micromolar (mediated by THTR-1) saturable thiamin uptake processes were affected by EPEC infection. Cell surface expression of hTHTR-1 and -2 proteins, (determined by the biotinylation method) showed a significantly (P < 0.01) lower expression in EPEC-treated cells compared with controls. EPEC infection also affected the steady-state mRNA levels as well as promoter activity of the SLC19A2 and SLC19A3 genes. Infecting Caco-2 cells with EPEC mutants that harbor mutations in the escN gene (which encodes a putative ATPase for the EPEC type III secretion system, TTSS) or the espA, espB, or espD genes (which encode structural components of the TTSS) did not affect thiamin uptake. On the other hand, mutations in espF and espH genes (which encode effector proteins) exhibited partial inhibition in thiamin uptake. These results demonstrate for the first time that EPEC infection of human intestinal epithelial cells leads to inhibition in thiamin uptake via effects on physiological and molecular parameters of hTHTR-1 and -2. Furthermore, the inhibition appears to be dependent on a functional TTSS of EPEC.
Figures








References
-
- Babei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 52: 2110–2120, 2003 - PubMed
-
- Berdanier CD. Micronutrients. In: Advanced Nutrition Boca Raton, FL: CRC, 1998
-
- Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol 290: G30–G35, 2006 - PubMed
-
- Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol 58: 946–958, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

