Hypotonicity-induced Renin exocytosis from juxtaglomerular cells requires aquaporin-1 and cyclooxygenase-2
- PMID: 19628672
- PMCID: PMC2754103
- DOI: 10.1681/ASN.2008090944
Hypotonicity-induced Renin exocytosis from juxtaglomerular cells requires aquaporin-1 and cyclooxygenase-2
Abstract
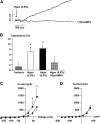
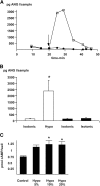
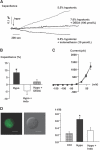
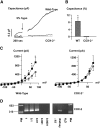
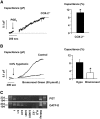
The mechanism by which extracellular hypotonicity stimulates release of renin from juxtaglomerular (JG) cells is unknown. We hypothesized that osmotically induced renin release depends on water movement through aquaporin-1 (AQP1) water channels and subsequent prostanoid formation. We recorded membrane capacitance (C(m)) by whole-cell patch clamp in single JG cells as an index of exocytosis. Hypotonicity increased C(m) significantly and enhanced outward current. Indomethacin, PLA(2) inhibition, and an antagonist of prostaglandin transport impaired the C(m) and current responses to hypotonicity. Hypotonicity also increased exocytosis as determined by a decrease in single JG cell quinacrine fluorescence in an indomethacin-sensitive manner. In single JG cells from COX-2(-/ -) and AQP1(-/ -) mice, hypotonicity increased neither C(m) nor outward current, but 0.1-muM PGE(2) increased both in these cells. A reduction in osmolality enhanced cAMP accumulation in JG cells but not in renin-producing As4.1 cells; only the former had detectable AQP1 expression. Inhibition of protein kinase A blocked the hypotonicity-induced C(m) and current response in JG cells. Taken together, our results show that a 5 to 7% decrease in extracellular tonicity leads to AQP1-mediated water influx in JG cells, PLA(2)/COX-2-mediated prostaglandin-dependent formation of cAMP, and activation of PKA, which promotes exocytosis of renin.
Figures

Similar articles
-
Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells.Am J Physiol Renal Physiol. 2005 Nov;289(5):F989-97. doi: 10.1152/ajprenal.00201.2005. Epub 2005 Jun 28. Am J Physiol Renal Physiol. 2005. PMID: 15985651
-
Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases.Circ Res. 2002 May 17;90(9):996-1003. doi: 10.1161/01.res.0000017622.25365.71. Circ Res. 2002. PMID: 12016266
-
Vesicle-associated membrane protein-2 (VAMP2) mediates cAMP-stimulated renin release in mouse juxtaglomerular cells.J Biol Chem. 2011 Aug 12;286(32):28608-18. doi: 10.1074/jbc.M111.225839. Epub 2011 Jun 27. J Biol Chem. 2011. PMID: 21708949 Free PMC article.
-
Regulation of renin secretion by renal juxtaglomerular cells.Pflugers Arch. 2013 Jan;465(1):25-37. doi: 10.1007/s00424-012-1126-7. Epub 2012 Jun 26. Pflugers Arch. 2013. PMID: 22733355 Review.
-
Exocytosis and endocytosis in juxtaglomerular cells.Acta Physiol Scand. 2000 Jan;168(1):95-9. doi: 10.1046/j.1365-201x.2000.00645.x. Acta Physiol Scand. 2000. PMID: 10691785 Review.
Cited by
-
Connexin Hemichannels Contribute to the Activation of cAMP Signaling Pathway and Renin Production.Int J Mol Sci. 2020 Jun 23;21(12):4462. doi: 10.3390/ijms21124462. Int J Mol Sci. 2020. PMID: 32585970 Free PMC article.
-
Genetic Variants of C-5312T REN Increased Renin Levels and Diastolic Blood Pressure Response to Angiotensin Receptor Blockers.Int J Hypertens. 2015;2015:930631. doi: 10.1155/2015/930631. Epub 2015 Oct 1. Int J Hypertens. 2015. PMID: 26495141 Free PMC article.
-
Experimental Evaluation of the Transport Mechanisms of PoIFN-α in Caco-2 Cells.Front Pharmacol. 2017 Nov 7;8:781. doi: 10.3389/fphar.2017.00781. eCollection 2017. Front Pharmacol. 2017. PMID: 29163167 Free PMC article.
-
Synthesis and secretion of renin in mice with induced genetic mutations.Kidney Int. 2012 Mar;81(6):529-38. doi: 10.1038/ki.2011.451. Epub 2012 Jan 18. Kidney Int. 2012. PMID: 22258323 Free PMC article. Review.
-
Comparison of the global gene expression of choroid plexus and meninges and associated vasculature under control conditions and after pronounced hyperthermia or amphetamine toxicity.BMC Genomics. 2013 Mar 5;14:147. doi: 10.1186/1471-2164-14-147. BMC Genomics. 2013. PMID: 23497014 Free PMC article.
References
-
- Skøtt O: Do osmotic forces play a role in renin secretion? Am J Physiol 255: F1–F10, 1988 - PubMed
-
- Kurtz A, Schweda F: Osmolarity-induced renin secretion from kidneys: evidence for readily releasable renin pools. Am J Physiol Renal Physiol 290: F797–F805, 2006 - PubMed
-
- Persson BE, Sakai T, Marsh DJ: Juxtaglomerular interstitial hypertonicity in Amphiuma: Tubular origin-TGF-signal. Am J Physiol 254: F445–F449, 1988 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

