Infiltrating CTLs in human glioblastoma establish immunological synapses with tumorigenic cells
- PMID: 19628762
- PMCID: PMC2716973
- DOI: 10.2353/ajpath.2009.081034
Infiltrating CTLs in human glioblastoma establish immunological synapses with tumorigenic cells
Abstract
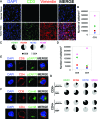
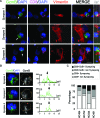
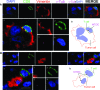
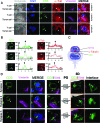
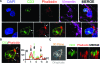
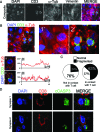
The immunological synapse between T cells and tumor cells is believed to be important for effective tumor clearance. However, the immunological synapse has never been imaged or analyzed in detail in human tissue. In this work, intercellular interactions between T cells and tumor cells were analyzed in detail in human glioblastoma. After characterization of the population of infiltrating T cells by multiple immunofluorescence staining and stereological quantification, the microanatomy of T cell-tumor cell intercellular communication was analyzed in detail using confocal microscopy and three-dimensional rendering. Cytotoxic T lymphocytes that infiltrated human glioblastoma underwent rearrangement when in contact with tumor cells, to form a three-dimensional structure in the intercellular contact area; this was characterized by microclusters of the CD3/TCR complex, re-arrangement of the cytoskeleton, and granzyme B polarization. In addition, such T cell-targeted cells show fragmentation of the microtubular system and increased expression levels of cleaved caspase 3, which suggests that cytotoxic T lymphocytes likely provoke changes in tumor cells and subsequently induce cell death. These results show that the formation of the cytotoxic T lymphocyte immunological synapse occurs in human tissue and may be relevant for the effective immune-mediated clearance of tumorigenic cells, therefore opening up new avenues for glioblastoma immunotherapy.
Figures


Similar articles
-
Imbalance of immunological synapse-kinapse states reflects tumor escape to immunity in glioblastoma.JCI Insight. 2018 Sep 20;3(18):e120757. doi: 10.1172/jci.insight.120757. eCollection 2018 Sep 20. JCI Insight. 2018. PMID: 30232280 Free PMC article.
-
Cx43-Gap Junctions Accumulate at the Cytotoxic Immunological Synapse Enabling Cytotoxic T Lymphocyte Melanoma Cell Killing.Int J Mol Sci. 2019 Sep 12;20(18):4509. doi: 10.3390/ijms20184509. Int J Mol Sci. 2019. PMID: 31547237 Free PMC article.
-
Effective immunotherapy of rat glioblastoma with prolonged intratumoral delivery of exogenous heat shock protein Hsp70.Int J Cancer. 2014 Nov 1;135(9):2118-28. doi: 10.1002/ijc.28858. Epub 2014 Apr 1. Int J Cancer. 2014. PMID: 24691976
-
Actin Cytoskeleton Straddling the Immunological Synapse between Cytotoxic Lymphocytes and Cancer Cells.Cells. 2019 May 16;8(5):463. doi: 10.3390/cells8050463. Cells. 2019. PMID: 31100864 Free PMC article. Review.
-
Actin remodeling and vesicular trafficking at the tumor cell side of the immunological synapse direct evasion from cytotoxic lymphocytes.Int Rev Cell Mol Biol. 2020;356:99-130. doi: 10.1016/bs.ircmb.2020.07.001. Epub 2020 Sep 9. Int Rev Cell Mol Biol. 2020. PMID: 33066877 Review. No abstract available.
Cited by
-
Molecular alterations in glioblastoma: potential targets for immunotherapy.Prog Mol Biol Transl Sci. 2011;98:187-234. doi: 10.1016/B978-0-12-385506-0.00005-3. Prog Mol Biol Transl Sci. 2011. PMID: 21199773 Free PMC article. Review.
-
The role of the mitochondria and the endoplasmic reticulum contact sites in the development of the immune responses.Cell Death Dis. 2018 Feb 28;9(3):336. doi: 10.1038/s41419-017-0237-7. Cell Death Dis. 2018. PMID: 29491398 Free PMC article. Review.
-
CCL2-expressing astrocytes mediate the extravasation of T lymphocytes in the brain. Evidence from patients with glioma and experimental models in vivo.PLoS One. 2012;7(2):e30762. doi: 10.1371/journal.pone.0030762. Epub 2012 Feb 2. PLoS One. 2012. PMID: 22319587 Free PMC article.
-
Imbalance of immunological synapse-kinapse states reflects tumor escape to immunity in glioblastoma.JCI Insight. 2018 Sep 20;3(18):e120757. doi: 10.1172/jci.insight.120757. eCollection 2018 Sep 20. JCI Insight. 2018. PMID: 30232280 Free PMC article.
-
Improved Treatment Efficacy of Antiangiogenic Therapy when Combined with Picornavirus Vaccination in the GL261 Glioma Model.Neurotherapeutics. 2016 Jan;13(1):226-36. doi: 10.1007/s13311-015-0407-1. Neurotherapeutics. 2016. PMID: 26620211 Free PMC article.
References
-
- Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17:87–94. - PubMed
-
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. - PubMed
-
- Stinchcombe JC, Griffiths GM. The role of the secretory immunological synapse in killing by CD8+ CTL. Semin Immunol. 2003;15:301–305. - PubMed
-
- Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, Xiong WD, Liu C, Kroeger K, Boyer O, Kupiec-Weglinski J, Klatzmann D, Castro MG, Lowenstein PR. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

