Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways
- PMID: 19628790
- PMCID: PMC2861358
- DOI: 10.1161/CIRCRESAHA.109.203083
Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways
Abstract
Rationale: The epicardium contributes to the majority of nonmyocardial cells in the adult heart. Recent studies have reported that the epicardium is derived from Nkx2.5-positive progenitors and can differentiate into cardiomyocytes. Not much is known about the relation between the myocardial and epicardial lineage during development, whereas insights into these embryonic mechanisms could facilitate the design of future regenerative strategies.
Objective: Acquiring insight into the signaling pathways involved in the lineage separation leading to the differentiation of myocardial and (pro)epicardial cells at the inflow of the developing heart.
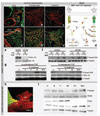
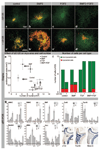
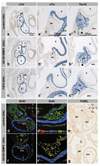
Methods and results: We made 3D reconstructions of Tbx18 gene expression patterns to give insight into the developing epicardium in relation to the developing myocardium. Next, using DiI tracing, we show that the (pro)epicardium separates from the same precursor pool as the inflow myocardium. In vitro, we show that this lineage separation is regulated by a crosstalk between bone morphogenetic protein (BMP) signaling and fibroblast growth factor (FGF) signaling. BMP signaling via Smad drives differentiation toward the myocardial lineage, which is inhibited by FGF signaling via mitogen-activated protein kinase kinase (Mek)1/2. Embryos exposed to recombinant FGF2 in vivo show enhanced epicardium formation, whereas a misbalance between FGF and BMP by Mek1/2 inhibition and BMP stimulation causes a developmental arrest of the epicardium and enhances myocardium formation at the inflow of the heart.
Conclusion: Our data show that FGF signaling via Mek1/2 is dominant over BMP signaling via Smad and is required to separate the epicardial lineage from precardiac mesoderm. Consequently, myocardial differentiation requires BMP signaling via Smad and inhibition of FGF signaling at the level of Mek1/2. These findings are of clinical interest for the development of regeneration-based therapies for heart disease.
Figures




Comment in
-
Look who's talking: FGFs and BMPs in the proepicardium.Circ Res. 2009 Aug 28;105(5):406-7. doi: 10.1161/CIRCRESAHA.109.205203. Circ Res. 2009. PMID: 19713545 Free PMC article. No abstract available.
References
-
- van den Hoff MJB, Kruithof BPT, Moorman AFM. Making more heart muscle. Bioessays. 2004;26:248–261. - PubMed
-
- Vliegen HW, van der LA, Cornelisse CJ, Eulderink F. Myocardial changes in pressure overload-induced left ventricular hypertrophy. A study on tissue composition, polyploidization and multinucleation. Eur Heart J. 1991;12:488–494. - PubMed
-
- Munoz-Chapuli R, Macias D, Gonzalez-Iriarte M, Carmona R, Atencia G, Perez-Pomares JM. The epicardium and epicardial-derived cells: multiple functions in cardiac development. Rev Esp Cardiol. 2002;55:1070–1082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

