Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential
- PMID: 19628804
- PMCID: PMC2754038
- DOI: 10.1161/STROKEAHA.109.556852
Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential
Abstract
Background and purpose: New treatment strategies for acute ischemic stroke must be evaluated in the context of effective reperfusion. Minocycline is a neuroprotective agent that inhibits proteolytic enzymes and therefore could potentially both inactivate the clot lysis effect and decrease the damaging effects of tissue-type plasminogen activator (t-PA). This study aimed to determine the effect of minocycline on t-PA clot lysis and t-PA-induced hemorrhage formation after ischemia.
Methods: Fibrinolytic and amidolytic activities of t-PA were investigated in vitro over a range of clinically relevant minocycline concentrations. A suture occlusion model of 3-hour temporary cerebral ischemia in rats treated with t-PA and 2 different minocycline regimens was used. Blood-brain barrier basal lamina components, matrix metalloproteinases (MMPs), hemorrhage formation, infarct size, edema, and behavior outcome were assessed.
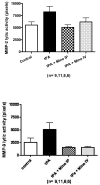
Results: Minocycline did not affect t-PA fibrinolysis. However, minocycline treatment at 3 mg/kg IV decreased total protein expression of both MMP-2 (P=0.0034) and MMP-9 (P=0.001 for 92 kDa and P=0.0084 for 87 kDa). It also decreased the incidence of hemorrhage (P=0.019), improved neurologic outcome (P=0.0001 for Bederson score and P=0.0391 for paw grasp test), and appeared to decrease mortality. MMP inhibition was associated with decreased degradation in collagen IV and laminin-alpha1 (P=0.0001).
Conclusions: Combination treatment with minocycline is beneficial in t-PA-treated animals and does not compromise clot lysis. These results also suggest that neurovascular protection by minocycline after stroke may involve direct protection of the blood-brain barrier during thrombolysis with t-PA.
Figures








References
-
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. - PubMed
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. - PubMed
-
- Ning M, Furie KL, Koroshetz WJ, Lee H, Barron M, Lederer M, Wang X, Zhu M, Sorensen AG, Lo EH, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66(10):1550–1555. - PubMed
-
- Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16(3):360–366. - PubMed
-
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19(6):624–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

