PARP-1 deficiency increases the severity of disease in a mouse model of multiple sclerosis
- PMID: 19628872
- PMCID: PMC2758007
- DOI: 10.1074/jbc.M109.013474
PARP-1 deficiency increases the severity of disease in a mouse model of multiple sclerosis
Abstract
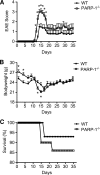
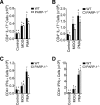
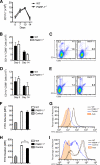
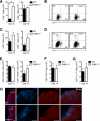
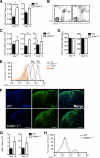
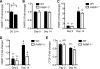
Poly(ADP-ribose) polymerase-1 (PARP-1) has been implicated in the pathogenesis of several central nervous system (CNS) disorders. However, the role of PARP-1 in autoimmune CNS injury remains poorly understood. Therefore, we studied experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis in mice with a targeted deletion of PARP-1. We identified inherent physiological abnormalities in the circulating and splenic immune composition between PARP-1(-/-) and wild type (WT) mice. Upon EAE induction, PARP-1(-/-) mice had an earlier onset and developed a more severe EAE compared with WT cohorts. Splenic response was significantly higher in PARP-1(-/-) mice largely because of B cell expansion. Although formation of Th1 and Th17 effector T lymphocytes was unaffected, PARP-1(-/-) mice had significantly earlier CD4+ T lymphocyte and macrophage infiltration into the CNS during EAE. However, we did not detect significant differences in cytokine profiles between PARP-1(-/-) and WT spinal cords at the peak of EAE. Expression analysis of different PARP isozymes in EAE spinal cords showed that PARP-1 was down-regulated in WT mice and that PARP-3 but not PARP-2 was dramatically up-regulated in both PARP-1(-/-) and WT mice, suggesting that these PARP isozymes could have distinct roles in different CNS pathologies. Together, our results indicate that PARP-1 plays an important role in regulating the physiological immune composition and in immune modulation during EAE; our finding identifies a new aspect of immune regulation by PARPs in autoimmune CNS pathology.
Figures



References
-
- Chambon P., Weill J. D., Mandel P. (1963) Biochem. Biophys. Res. Commun. 11, 39–43 - PubMed
-
- Kim M. Y., Zhang T., Kraus W. L. (2005) Genes Dev. 19, 1951–1967 - PubMed
-
- Amé J. C., Spenlehauer C., de Murcia G. (2004) BioEssays 26, 882–893 - PubMed
-
- Moroni F. (2008) Curr. Opin. Pharmacol. 8, 96–103 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

